Production method of 4, 4-diaminodiphenyl ether
A technology of diaminodiphenyl ether and dinitrodiphenyl ether, which is applied in the field of hydrogenation synthesis of aromatic nitro compounds, can solve the problems of poor chromaticity, low yield, high energy consumption and the like, and achieves improved purity and improved Product focus, avoid the effect of darkening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A production method of 4,4'-diaminodiphenyl ether, comprising the following steps:
[0039] ①Add 1kg 4,4'-dinitrodiphenyl ether, 5g catalyst Pt / C and 5kg methanol into the autoclave, stir and mix evenly, steam 500g methanol by low-temperature vacuuming, and replace the autoclave with nitrogen 3 times , and then fill the autoclave with hydrogen, the hydrogenation pressure is 0.4MPa, and react at 50 ° C for 6 hours to obtain a reaction solution;
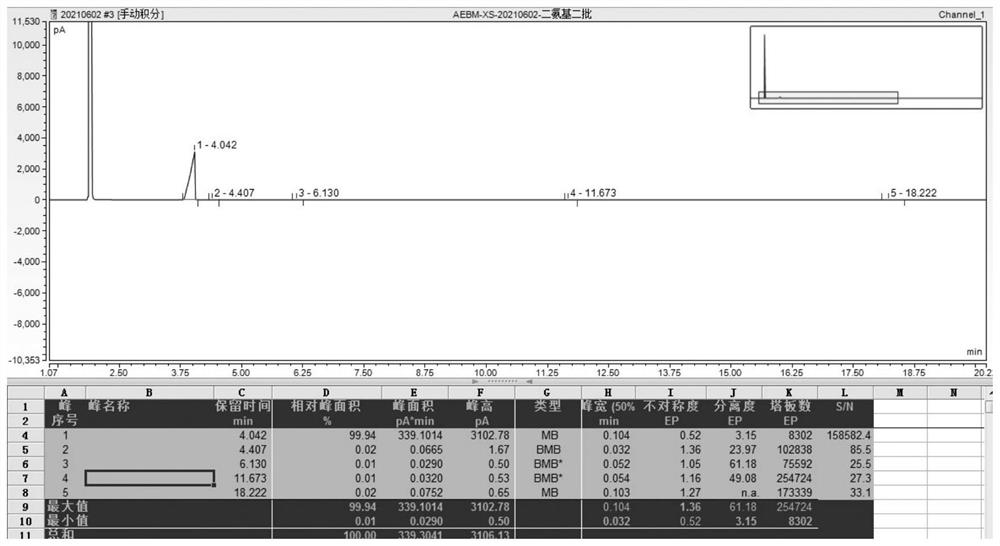
[0040] ②Add 5g of sodium thiosulfate and 2.5kg of deionized water to the crystallizer, and use nitrogen to remove the air, and then filter the reaction solution obtained in step ① into the crystallizer for 10 hours of crystallization, and the crystallization temperature is 45 ℃, after the crystallization is completed, filter and wash the filter cake with deionized water to obtain 738 g of white granular product 4,4'-diaminodiphenyl ether, the product purity is 99.72%, and the particle size is about 60 mesh.
Embodiment 2
[0042] A production method of 4,4'-diaminodiphenyl ether, comprising the following steps:
[0043] ①Add 1kg of 4,4'-dinitrodiphenyl ether, 20g of catalyst Pd / C and 6kg of toluene into the autoclave, stir and mix evenly, steam 1kg of hydrogenation solvent by low-temperature vacuuming, and replace the autoclave with nitrogen 5 times, then fill the autoclave with hydrogen, the hydrogenation pressure is 1.2MPa, and react at 90 ° C for 4 hours to obtain a reaction solution;
[0044] ②Add 60g of zinc powder and 12kg of deoxidized ethylene glycol into the crystallizer, and use nitrogen to remove the air, then filter the reaction solution obtained in step ① and enter the crystallizer for crystallization for 15 hours. The crystallization temperature is 60°C, and the crystallization After completion, filter and wash the filter cake to obtain 742g of white granular product 4,4'-diaminodiphenyl ether, the yield is 96.48%, the product purity is 99.80%, and the particle size is about 150 me...
Embodiment 3
[0046] A production method of 4,4'-diaminodiphenyl ether, comprising the following steps:
[0047] ①Add 1kg 4,4'-dinitrodiphenyl ether, 10g catalyst Raney nickel and 5.4kg hydrogenation solvent N,N-dimethylacetamide into the autoclave, stir and mix evenly, and use low temperature vacuuming Steamed out 0.8kg of hydrogenation solvent, replaced the autoclave with nitrogen 4 times, then filled the autoclave with hydrogen, the hydrogenation pressure was 0.6MPa, and reacted at 65°C for 4.5 hours to obtain a reaction solution;
[0048] ②Add 21.6g of hydroxylamine hydrochloride and 4.32kg of deoxygenated chlorobenzene to the crystallizer, and use nitrogen to remove air, then filter the reaction solution obtained in step ① and enter the crystallizer for crystallization for 12 hours, and the crystallization temperature is 55°C, After the crystallization is completed, filter and wash the filter cake to obtain 750 g of white granular product 4,4'-diaminodiphenyl ether, the yield is 97.53%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com