Recombinant leukocyte inhibitory factor and leech peptide chimeric protein mutant
A technology of inhibitory factors and chimeric proteins, which is applied in the field of molecular biology, can solve the problems of easy mismatch, lower protein purity, and lower biological activity, so as to reduce the probability of mismatch, improve protein purity, and reduce isomers The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 Acquisition of TNHH gene mutants
[0107] 1. Primer synthesis
[0108] According to the mutation sites of TNHH shown in Table 1, Primer software was used to design the primers required for mutation, and Shanghai Sangon Engineering was entrusted to design and synthesize them. See 2 for primers required for TNHH mutants.
[0109] Table 1 Mutation sites of different TNHH mutants
[0110] mutant Mutation site TNHH-M1 278 Pro→Thr, 282 Leu→Glu, 162, 214 Cys→Ala TNHH-M2 274th Phe→Ser, 277th Ile→Gly, 162nd, 214th Cys→Ala TNHH-M3 278 Pro→Thr, 282 Leu→Glu, 211, 214 Cys→Ala TNHH-M4 274th Phe→Ser, 277th Ile→Gly, 211th, 214th Cys→Ala
[0111] Table 2 Primers required for TNHH mutants
[0112]
[0113]
[0114] 2. PCR reaction
[0115] The primers required to mutate the 162nd amino acid of the original TNHH sequence, the template DNA and the buffer system were mixed according to the following reaction system.
[0116] ...
Embodiment 2
[0150] Example 2 Shake flask expression of mutant pET-3c-TNHH / BL21(DE3)pLysS-M1~pET-3c-TNHH / BL21(DE3)pLysS-M4 strains
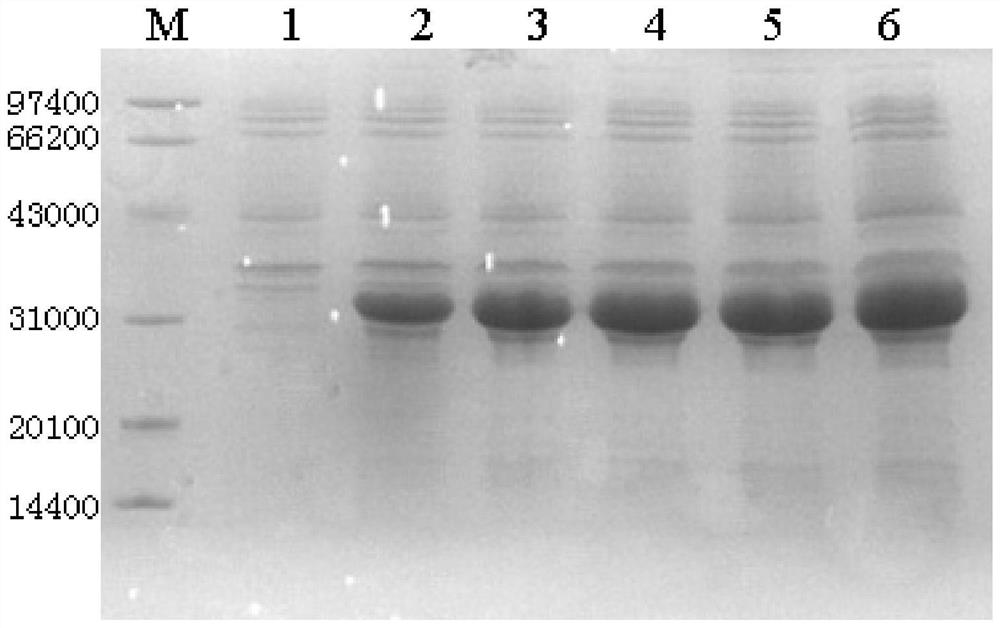
[0151] The four mutant strains and the original E. coli strain pET-3c-TNHH / BL21(DE3)pLysS were used for preliminary expression studies on shake flasks. First, pick a single clone from the corresponding plate of the strain and inoculate it into LB ampicillin medium (0.5% yeast powder, 1% peptone, 1% sodium chloride, 100 mg / L ampicillin), inoculate 2 bottles per strain, and cultivate at 37 °C for 10 h. OD 600 Between 0.8 and 1.2, use a final concentration of 0.4 mol / LIPTG to induce expression, and the induction for 4 hours ends. The bacterial solution was centrifuged at 12,000 rpm for 3 min to discard the supernatant, and its expression was analyzed by SDS-PADE. The results are shown in figure 1 ,from figure 1 It can be seen that the protein expression level after gene mutation is increased compared with the initial TNHH expression level.
Embodiment 3
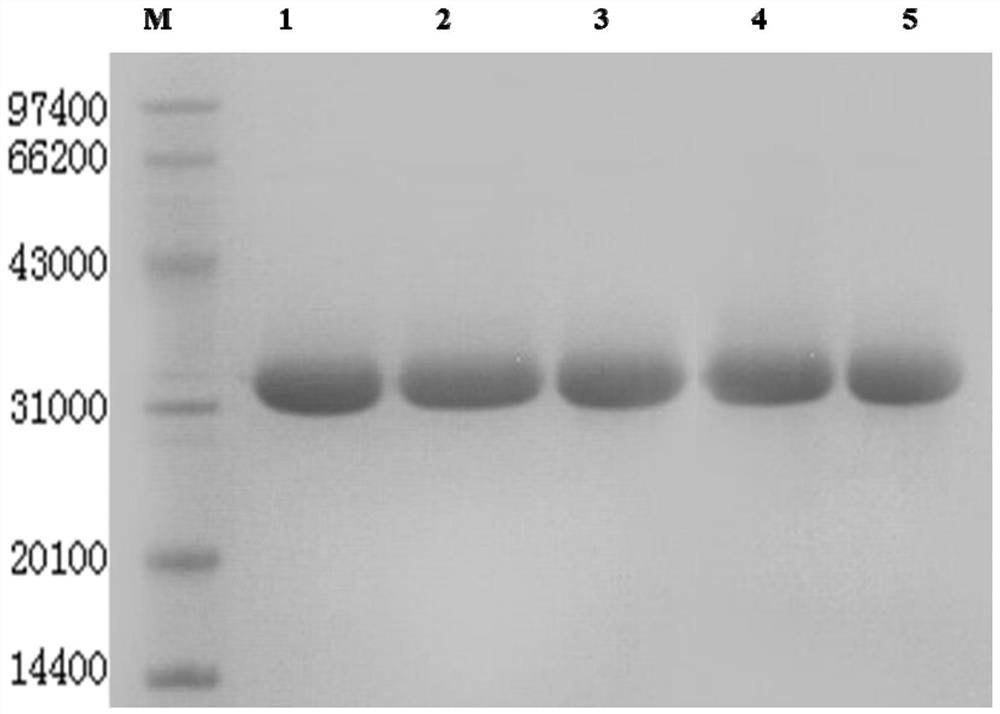
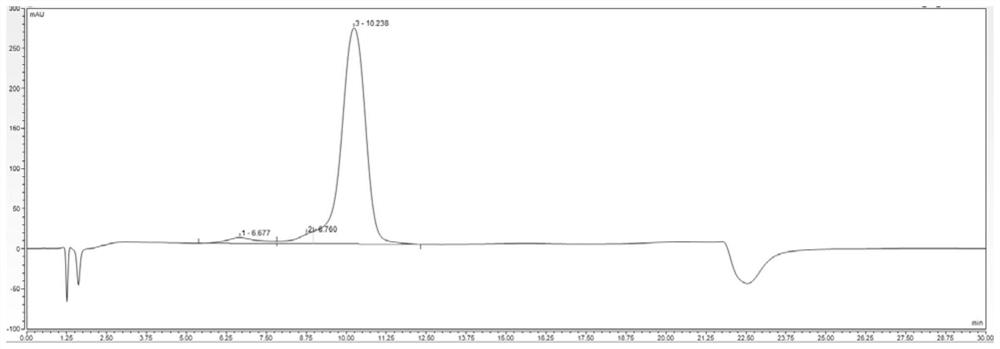
[0152] Example 3 Fermentation and purification of TNHH and mutants TNHHM1-M4
[0153] 1. Fermentation induction of original strain pET-3c-TNHH / BL21(DE3)pLysS and mutant strain pET-3c-TNHH / BL21(DE3)pLysS-M1~pET-3c-TNHH / BL21(DE3)pLysS-M4 Express
[0154] 1. Seed cultivation:
[0155] Pick the original strain pET-3c-TNHH / BL21(DE3)pLysS, and the mutant strain pET-3c-TNHH / BL21(DE3)pLysS-M1~pET-3c-TNHH / BL21(DE3)pLysS-M4 single colonies respectively It was inoculated into LB medium containing 100 mg / L ampicillin, and cultured at 37° C. and 150 rpm for 10 h to obtain seed liquid for fermentation.
[0156] 2. 30L fermentation tank fermentation:
[0157] Add 10L fermentation base medium (0.3% yeast powder, 0.5% peptone, 3.5% dodecahydrate and disodium hydrogen phosphate, 0.5% potassium dihydrogen phosphate, 0.1% ammonium chloride, 0.1% sodium chloride, 0.1% magnesium sulfate, 1% glucose), autoclaved, after the medium was cooled to 37°C, the pH was adjusted to 7.0 with ammonia water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com