Diacerein capsule with high bioavailability and preparation method thereof
A technology for diacerein and capsules, applied in the field of pharmaceutical preparations, can solve problems such as reduced bioavailability, achieve the effects of improving bioavailability, inhibiting recrystallization, and shortening production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
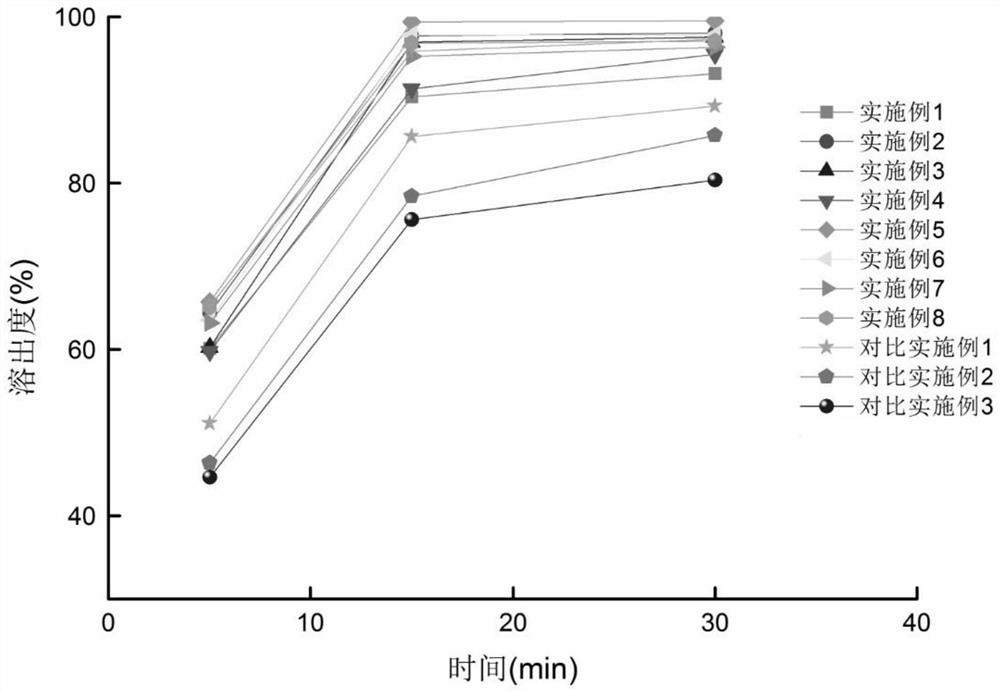
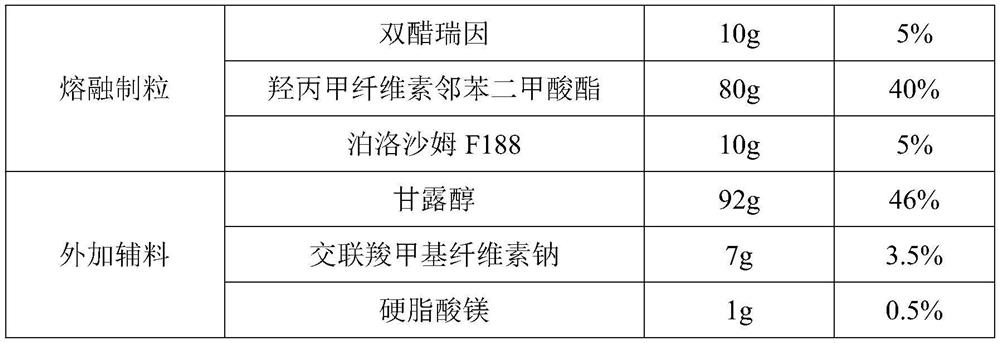
Embodiment 1
[0029] (1) Prescription
[0030]
[0031] (2) Preparation process
[0032] The prescription amount of diacerein, hypromellose phthalate and poloxamer F188 are mixed and then melt granulated.
[0033] Hot melt extrusion parameters: temperature setting: 80℃ / 120℃ / 160℃ / 160℃ / 160℃ / 160℃ / 160℃ / 160℃; screw speed: 150rpm; feeding speed: 2kg / h.
[0034] After crushing, pass through an 80-mesh sieve, mix with mannitol, croscarmellose sodium, and magnesium stearate evenly, then fill in capsules, package, and inspect to obtain a finished product.
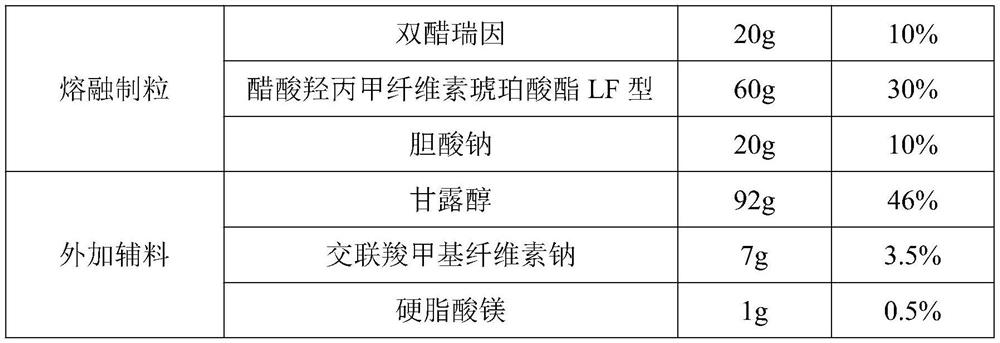
Embodiment 2
[0036] (1) Prescription
[0037]
[0038] (2) Preparation process
[0039] The prescription amount of diacerein, hypromellose acetate succinate LF type and sodium cholate are mixed and then melt granulated.
[0040] Hot melt extrusion parameters: temperature setting: 120℃ / 150℃ / 170℃ / 170℃ / 170℃ / 170℃ / 170℃ / 170℃; screw speed: 100rpm; feeding speed: 2kg / h.
[0041] After crushing, pass through an 80-mesh sieve, mix with mannitol, croscarmellose sodium, and magnesium stearate evenly, then fill in capsules, package, and inspect to obtain a finished product.
Embodiment 3
[0043] (1) Prescription
[0044]
[0045] (2) Preparation process
[0046] The diacerein in the prescribed amount, acrylic resin L100-55 type and Tween 80 are mixed and then melted and granulated.
[0047] Hot melt extrusion parameters: temperature setting: 120℃ / 150℃ / 175℃ / 175℃ / 175℃ / 175℃ / 175℃ / 175℃; screw speed: 120rpm; feeding speed: 2kg / h.
[0048] After crushing, pass through an 80-mesh sieve, mix with microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and magnesium stearate, and then fill in capsules, package, and inspect to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com