Isoniazide hapten derivative, isoniazide artificial antigen, hybridoma cell strain, isoniazide monoclonal antibody and application
A monoclonal antibody and artificial antigen technology, applied in microorganisms, biological testing, animal/human peptides, etc., which can solve the problems of antibody cross-reaction, destruction of characteristic differential groups, and difficulty in determination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention provides the preparation method of the isoniazid hapten derivative described in the above technical scheme, comprising the following steps:
[0040] Isoniazid and acetone are subjected to condensation reaction under alkaline conditions to obtain intermediate 1;
[0041] Mix the intermediate 1, monomethyl succinate, persulfate, trifluoroacetic acid, metal salt catalyst and water, and carry out oxidative decarboxylation reaction to obtain intermediate 2;
[0042] The intermediate 2 is acidified after being subjected to an alkaline hydrolysis reaction to obtain the isoniazid hapten derivative;
[0043] .
[0044] In the present invention, unless otherwise specified, the raw materials used are all commercially available products well known to those skilled in the art.
[0045] The isoniazid and acetone of the present invention undergo a condensation reaction under alkaline conditions to obtain intermediate 1.
[0046] In the present invention, the...
Embodiment 1
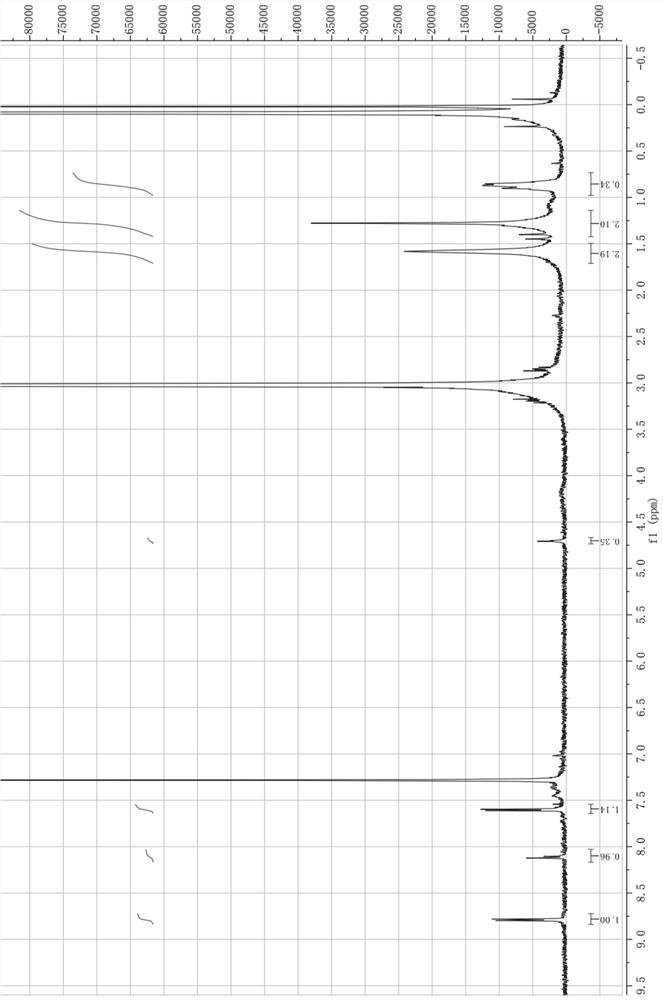
[0125] Preparation of isoniazid hapten derivative with structure shown in formula I
[0126]
[0127]
[0128]
[0129] 13.7 g of isoniazid and 40 mL of acetone were added to the NaOH aqueous solution (NaOH mass 5 g, 1 mol / L) under stirring conditions, and the reaction was stirred at 50 °C for 2 h. At the end of the reaction, the excess acetone was evaporated under reduced pressure, 20 mL of water was added, the temperature was kept at 12 °C for crystallization for 2 h, filtered, and the obtained solid powder was vacuum-dried at 45 °C to obtain Intermediate 1.
[0130] To 10.0 g of Intermediate 1 was added 300 mL of water, 64.0 g of ammonium persulfate, 6.5 g of TFA and 20 g of AgNO 3 , react at room temperature (25°C) for 2 h, add 30 mL of ethyl acetate and let stand for layers, take the organic layer and concentrate under reduced pressure to evaporate the ethyl acetate to obtain intermediate 2.
[0131] Add 50 mL of water to intermediate 2, then dropwise add NaOH (...
Embodiment 2
[0134] Preparation of isoniazid artificial antigen
[0135] Take 10 mg of the isoniazid hapten derivative prepared in Example 1, add 1 mL of DMSO to dissolve completely, and obtain the isoniazid hapten derivative solution;
[0136] Weigh 10 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, dissolve it in 100 μL of water, add it to the isoniazid hapten derivative solution, and react at room temperature for 1 h. A cross-linked isoniazid hapten derivative solution is obtained;
[0137] Weigh 20 mg of bovine thyroglobulin and dissolve it in 5 mL of PBS to obtain a bovine thyroglobulin solution;
[0138] Mix the cross-linked isoniazid hapten derivative solution and bovine thyroglobulin solution, stir at room temperature for 2 hours to obtain a composite reaction solution, and dialyze the composite reaction solution with PBS buffer (pH=7.4) (dialysis bag 7000Da) 4 times to obtain The isoniazid artificial antigen having the structure shown in formula II, the isonia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com