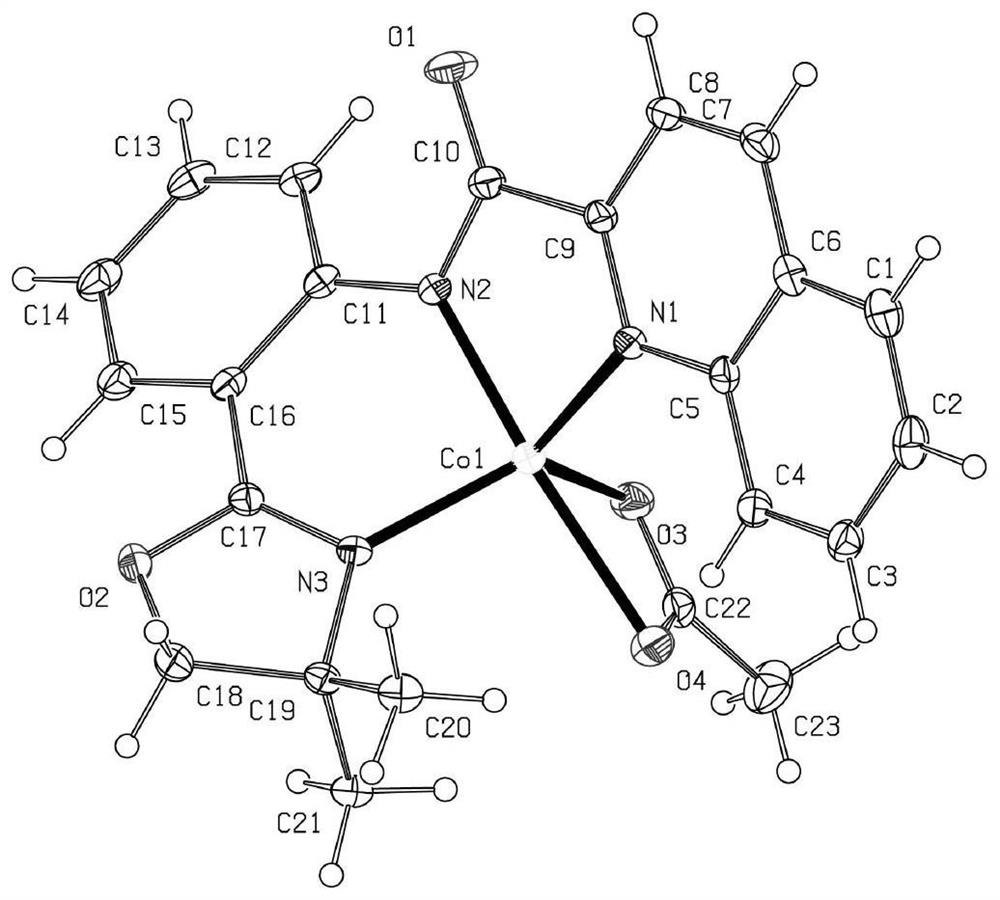
Method for synthesizing high-regioselectivity alpha-alkenyl stannane through hydrogenation of terminal alkyne Martensite tin
A terminal alkyne, alkenylstannane technology, applied in the field of α-alkenylstannane, can solve problems such as the decline of regioselectivity, and achieve the effects of simple operation, mild reaction conditions and increased practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Method and application of cobalt-catalyzed terminal alkyne martensitine hydrogenation to synthesize α-alkenylstannane
[0097]Add Co(OAc) to the flat-bottomed vial sequentially in a glove box at room temperature under nitrogen. 2 (0.012 mol, 2 mol %), OPQC (0.0144 mmol, 2.4 mol %), tetrahydrofuran (THF) (1.2 mL), and stirred at room temperature for 0.5 hr. Subsequently, tributyltin hydride (0.72 mmol) shown in formula II and alkyne (0.6 mmol) shown in formula I were successively added, and after stirring at room temperature for 1 hour, column chromatography separation (eluting solvent was petroleum ether or mixture of petroleum ether and ethyl acetate) to give the product. (In individual reaction conditions, Co(OAc) 2 , OPQC, reaction times and amounts of reactants vary and are specified under each product. )
[0098]
[0099] Tributyl(1-phenylvinyl)stannane
[0100] Tributyl(1-phenylvinyl)stannane
[0101]
[0102] Oily liquid, 90% yield, regiose...
Embodiment 2
[0371] Example 2: Product Synthesis of α-Alkenyl Azides (Application Example 1)
[0372] Under air, to a round bottom flask were added III (0.20 mmol), methanol (MeOH) (2 mL), Cu(OAc) sequentially 2 ·H 2 O (0.2 mmol), sodium azide (0.4 mmol) and the reaction were stirred at room temperature for 16 hours. After the completion of the reaction, silica gel was filtered, and ether (20 mL×3) was used as the eluent. Then, spin off the solvent, perform silica gel column chromatography, and elute with PE to obtain the target product XIII.
[0373] In formula III, R 1 , R 2 is defined as above.
[0374]
[0375] XIII-1:
[0376] 1-(Azidovinyl)benzene
[0377] 1-(Azidovinyl)benzene
[0378]
[0379] Colorless oily liquid, 0.0264 g, 91% yield. 1 H NMR: (400MHz, CDCl 3 )δ7.60-7.52(m, 2H), 7.40-7.30(m, 3H), 5.43(d, J=2.0Hz, 1H), 4.96(d, J=2.0Hz, 1H); 13 C NMR: (100MHz, CDCl 3 ) δ 145.0, 134.2, 129.1, 128.4, 125.5, 98.0. Known compounds, consistent with the data in the lite...
Embodiment 3
[0380] Embodiment 3: product synthesis allyl alcohol compound (application example two)
[0381]
[0382] In formula III, R 1 , R 2 is defined as above.
[0383] XIV-1
[0384] 2-Methyl-3-phenylbut-3-en-2-ol
[0385] 2-Methyl-3-phenylbut-3-en-2-ol
[0386]
[0387] Prepared according to known literature J. Org. Chem. 1987, 52, 4868-4874. Under nitrogen, III-1 (0.0786 g, 0.20 mmol), THF (tetrahydrofuran) (2.0 mL), and methyllithium (125 μL, 1.6 M of diethoxy) were added dropwise to a dry 25 mL reaction tube at 0°C. base methane solution, 0.20 mmol), and reacted at 0 °C for 0.5 hours. The color of the reaction solution gradually deepened. Subsequently, acetone (0.0120 g, 0.20 mmol) was added dropwise at 0° C., and the reaction solution was returned to room temperature for 16 hours. After the completion of the reaction, silica gel was filtered, and ether (20 mL×3) was used as the eluent. Then, spin off the solvent, and pass through the column with PE (petroleum eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com