Recombinant human fusion collagen as well as efficient hydroxylation method and application thereof
A collagen and hydroxylation technology, applied in the field of genetic engineering, can solve the problems of reporting and achieve high biological activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
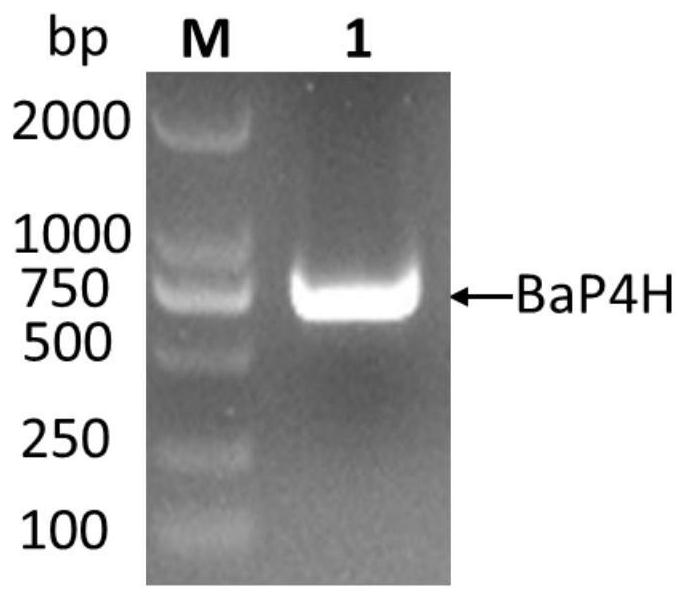
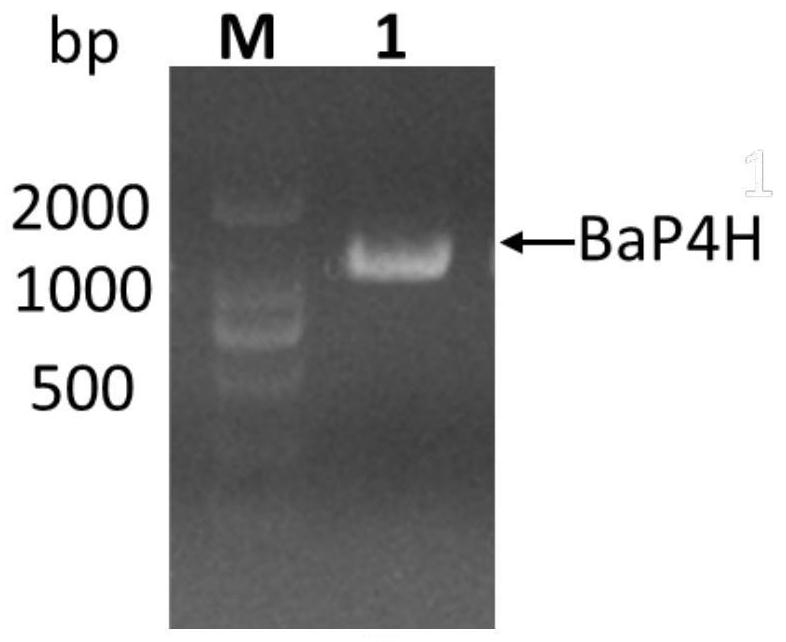
[0073] According to the amino acid sequence SEQ ID No. 1 of the recombinant human fusion collagen, according to the codon preference expressed by the host E. coli, the NdeI and BamHI restriction sites were avoided during the design process, and the gene sequence was optimized. The optimized gene sequence is SEQ ID As shown in No. 2, in the same way, the amino acid sequence of Bacillus anthracis hydroxylase (BaP4H) was reversely designed to obtain its gene sequence, and the two gene sequences were handed over to Shanghai Sangon Biological Co., Ltd. for full gene synthesis. The recombinant collagen gene sequence is directly connected between the restriction sites NdeI and BamHI of the expression vector pET28a to obtain the recombinant vector pET28a-rhCOL(I-III); the Bacillus anthracis hydroxylase (BaP4H) gene sequence is connected to the vector On pUC57, the recombinant vector pUC57-BaP4H was obtained.
[0074] Construction of recombinant expression vector pGro7-BaP4H, after ins...
Embodiment 2
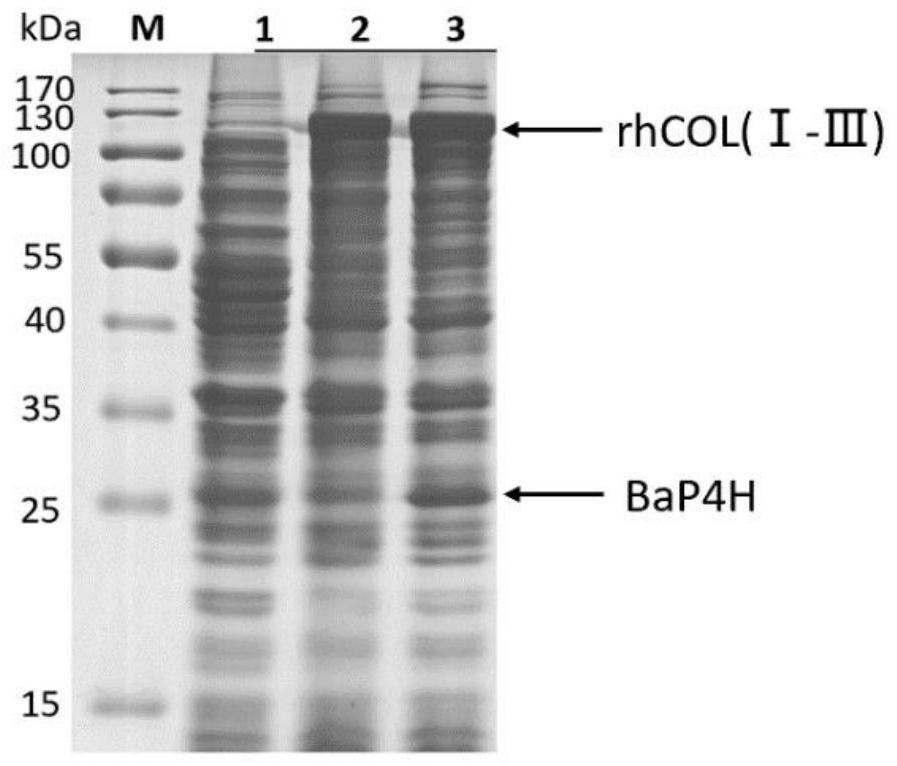
[0096] Example 2: Co-expression and purification of recombinant human fusion collagen and proline hydroxylase in E. coli host
[0097] Co-expression conditions were optimized, including medium composition, induction temperature, inducer concentration, IPTG addition time, etc., and a large amount of expression was carried out under optimal conditions. The optimal expression conditions were as follows:
[0098] (1) Seed liquid preparation: pick a single colony on the transformation plate in Example 1 on the ultra-clean workbench and inoculate it in 10 mL of LB liquid medium (Cm + and Kan + ), cultured at 37°C, 220rpm in a constant temperature shaker for 10-12h as a seed solution.
[0099] (2) Co-expression: The above-mentioned seed solution was transferred to 3 shake flasks (Cm + and Kan + ), where no inducer was added to the No. 1 shake flask, and the No. 3 shake flask was added with an arabinose solution with a final concentration of 2 mg / mL before inoculation to induce BaP...
Embodiment 3
[0107] Example 3: Structure Detection of Recombinant Human Fusion Collagen
[0108] (1) Analysis of amino acid composition
[0109] The BCA method was used to measure the protein concentration after ultrafiltration and concentration using a protein concentration assay kit (Thermo Fisher). Take a 10 mg protein sample, add 5 mL of 6M hydrochloric acid into a hydrolysis tube, vacuumize and fill with nitrogen, and place at 110 °C The oven was hydrolyzed for 24h. After hydrolysis, the hydrolysis tube was cooled to room temperature, and ddH was used. 2 O dilute to 25mL, take 2mL nitrogen to dry (add a small amount of ddH 2 O was dried twice), and finally 1 mL of pH2.2, 0.02M hydrochloric acid buffer was added to resuspend, filtered with a 0.45 μm membrane filter, and detected by Hitachi L8900 amino acid automatic analyzer (Hitachi Co., Ltd., Japan).
[0110] Its amino acid analysis map such as Figure 5 As shown, the hydroxylation of recombinant collagen was successfully achieved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com