Anode material--lithium nickelate cobalt preparation method for lithium ion battery
A technology for lithium-ion batteries and cathode materials, applied in electrode manufacturing, battery electrodes, active material electrodes, etc., can solve the problem that the hydrothermal method is not suitable for large-scale industrial production, it is difficult to adjust the particle size distribution of products, and it is difficult to obtain ion precursors, etc. problems, achieve excellent layered structure, ensure electrochemical performance, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
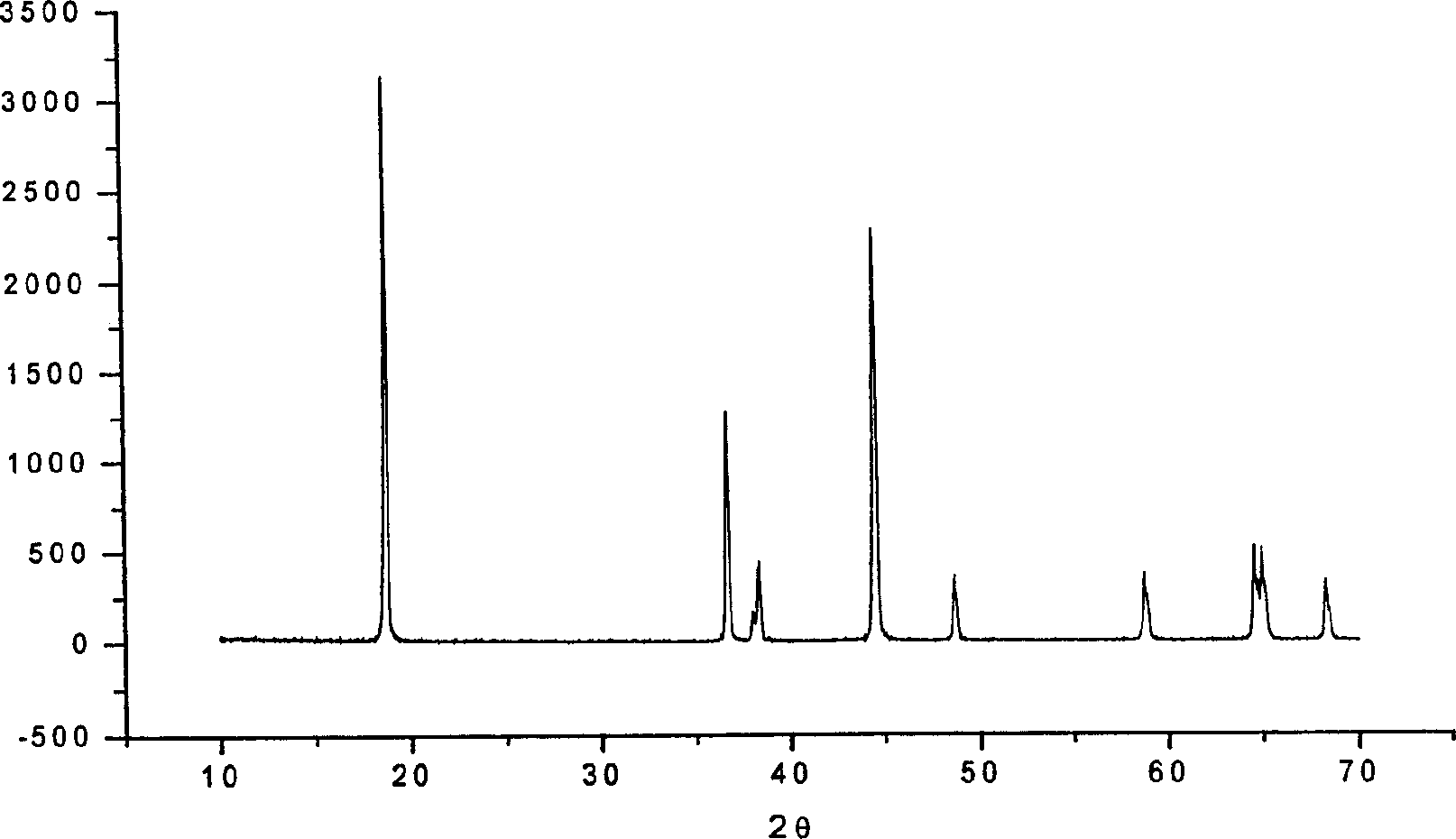
Image
Examples
Embodiment 1
[0039] Take 405.3g Li 2 SO 4 , 108.7g CoSO 4 , 411.24g NiSO 4 1. Dissolve 135g of tartaric acid in 1L of water, add an appropriate amount of gelatin into the solution, raise the temperature to 45°C, and continue adding gelatin until the viscosity of the solution reaches 200-400mPa.sec. Keep the temperature at 45°C for 30 minutes with slight stirring. Turn on the spray drying device, adjust the rotational speed of the centrifugal atomizer to 14-16m / s, start spray drying, collect the obtained precursor, and perform solid-phase sintering according to the following program control method, the first stage: 350°C, 2h; the second Stage: 400℃~500℃~600℃, 2~2~2h; the third stage: 800℃, 10h~12h, the whole process adopts oxygen atmosphere.
Embodiment 2
[0041] Take 243.17g CH3 COOLi, 110.15g (CH 3 COO) 2 Co, 496g (CH 3 COO) 2 Dissolve Ni and 172.8g citric acid in 1L water, add an appropriate amount of gelatin into the solution, raise the temperature to 45°C, and continue adding gelatin until the solution viscosity reaches 200-400mPa.sec. Keep the temperature at 45°C for 30 minutes with slight stirring. Turn on the spray drying device, adjust the rotational speed of the centrifugal atomizer to 16-18m / s, start spray drying, collect the obtained precursor, and perform solid-phase sintering according to the following programmed method, the first stage: 380°C, 1.5h; The second stage: 400~500~600°C, 2h~2h~2h; the third stage: 800°C, 10~12h, the whole process adopts oxygen atmosphere.
Embodiment 3
[0043] Take 243.17g CH 3 COOLi, 90.5g (CH 3 COO) 2 Co, 506g (CH 3 COO) 2 Ni,, 135g of tartaric acid in 1L of water, add an appropriate amount of gelatin to the solution, raise the temperature to 45°C, continue to add gelatin until the solution viscosity reaches 200-400mPa.sec. Keep the temperature at 45°C for 30 minutes with slight stirring. Turn on the spray drying device, adjust the rotational speed of the centrifugal atomizer to 16-18m / s, start spray drying, collect the obtained precursor, and carry out solid-phase sintering according to the following program control method, the first stage: 300°C, 1.5h; The second stage: 400~500~600°C, 2h~2h~2h; the third stage: 850°C, 10~12h, the whole process adopts oxygen atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com