Ethanol ammoxidizing process to synthesize high-purity acetonitrile
A technology for oxidative synthesis and ethanol ammonia is applied in the field of manufacture of aliphatic nitrile or carbocyclic and heterocyclic compound side chain nitriles, and can solve the problems of unsatisfactory acetonitrile yield, unstable product quality, and low acetonitrile recovery rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
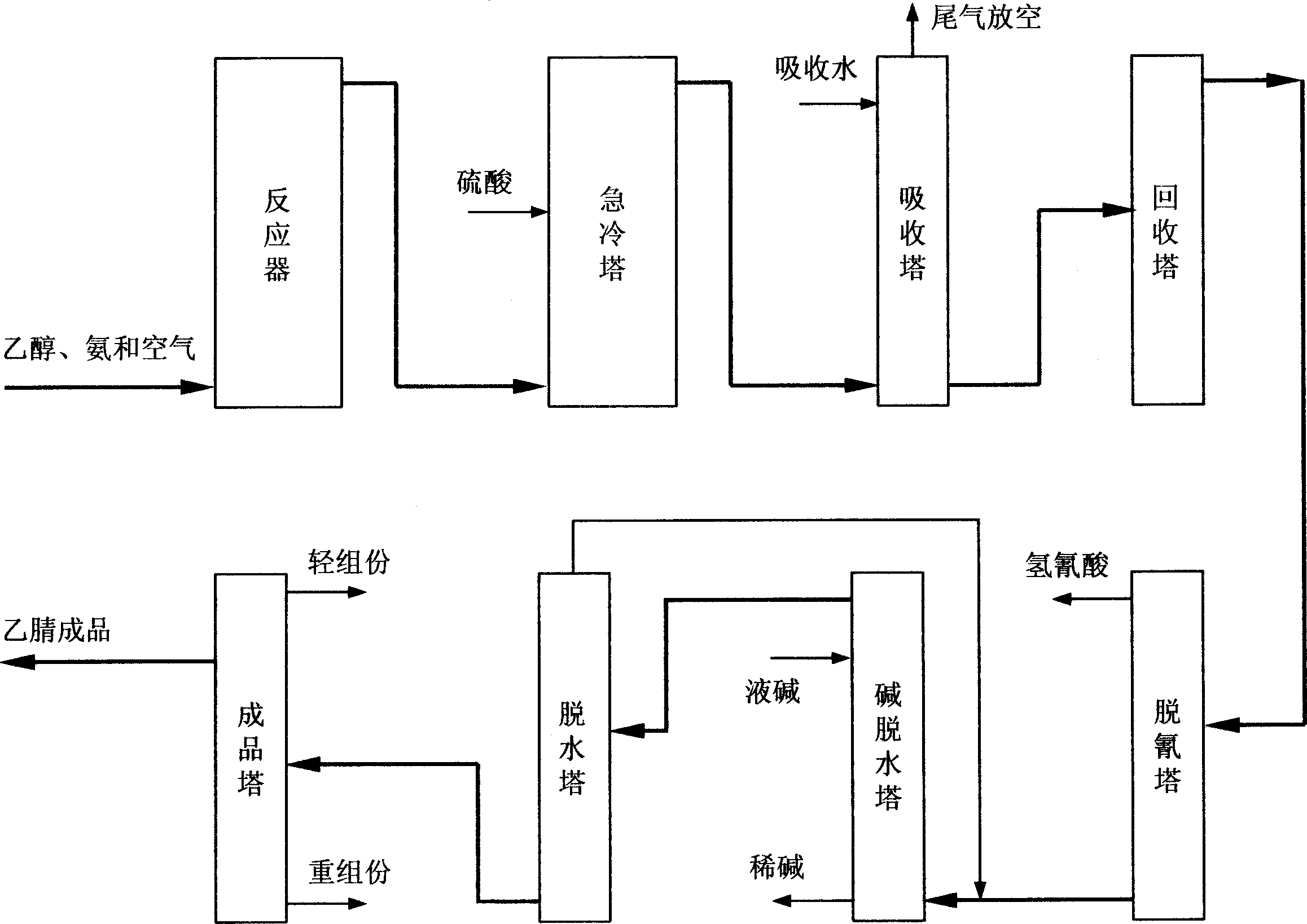
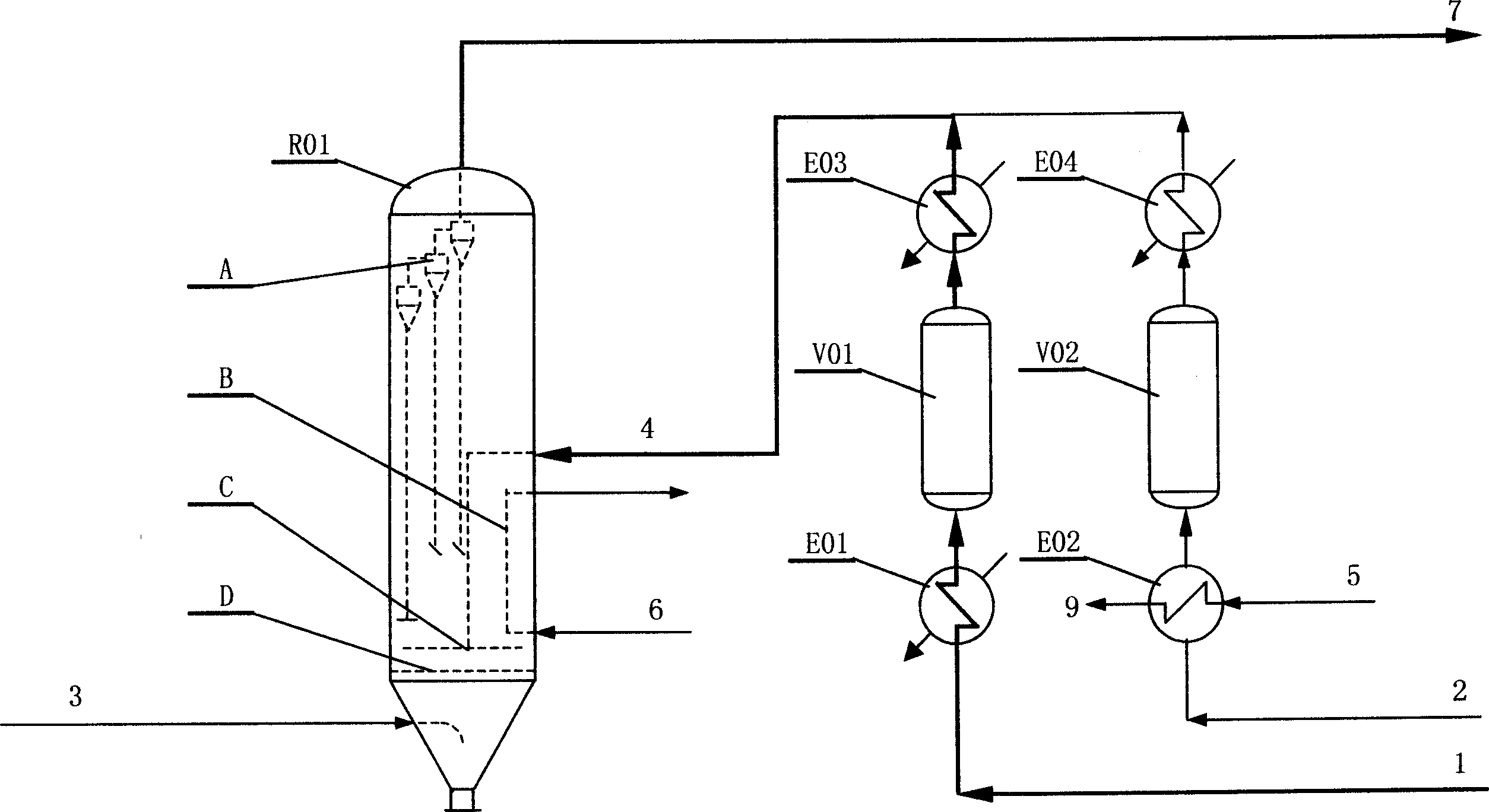
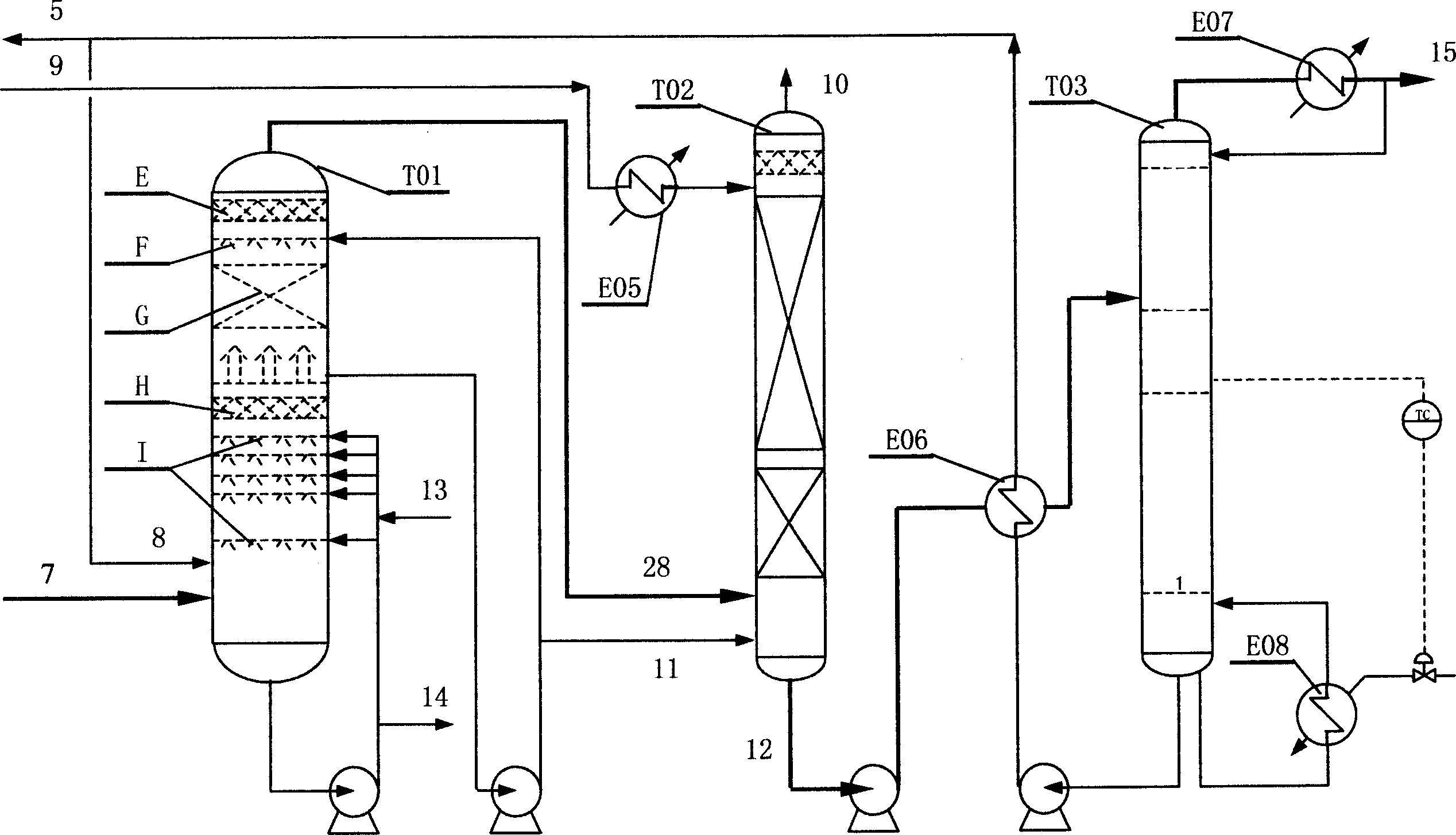
[0102] The raw materials ethanol, ammonia and air in the gas phase are under the action of the ethanol ammoxidation catalyst in the fluidized bed reactor to produce acetonitrile as the main product and hydrogen cyanide as a by-product. The reaction gas mixture goes through a series of treatment and separation processes such as two-stage quench tower, packed absorption tower, plate recovery tower, decyanation tower, alkali dehydration tower, packed dehydration tower and finished product tower, and finally obtains the finished acetonitrile , while by-product sodium cyanide.
[0103] 1 reaction process
[0104] The liquid raw materials ethanol and ammonia are respectively vaporized and preheated to 130°C. After the two raw materials are mixed, they enter the reactor from the middle of the reactor. The lower part enters the reaction bed; the reaction raw material air from the air compressor enters from the bottom of the reactor, is evenly distributed through the air distribution ...
Embodiment 2
[0122] In the reaction process, ethanol and liquid ammonia are vaporized and preheated to 140-150°C respectively; the ammoxidation reaction is at 400-450°C; the pressure at the top of the reactor is 0.040-0.060Mpa gauge pressure; the linear velocity of the empty bed is 0.6-0.7m / s; Reaction weight hourly space velocity WWH0.15~0.2h -1 ;
[0123] In the recovery process, the spray liquid of the quenching tower is adjusted to pH 1~2, and the reaction gas is further cooled to 40~50°C; the temperature of the absorption water in the absorption tower is 5~7°C, and the mass flow ratio to acetonitrile is 17~22; the recovery tower absorbs The liquid is heated to 80-90°C; the top of the tower adopts external reflux, and the reflux ratio is 4.0-5.0. The sensitive point temperature control range of the stripping section is 90-95°C;
[0124] In the refining process, the reflux ratio at the top of the decyanation tower is 5.0-6.0, and the operating pressure is 0.08-0.10Mpa absolute pressure...
Embodiment 3
[0127] In the reaction process, ethanol and liquid ammonia are respectively vaporized and preheated to 90-100°C; the ammoxidation reaction is at 350-400°C; the pressure at the top of the reactor is 0.025-0.030Mpa gauge pressure; the linear velocity of the empty bed is 0.4-0.5m / s; Reaction weight hourly space velocity WWH0.10~0.15h -1 ;
[0128] In the recovery process, the spray liquid of the quenching tower is adjusted to pH 4-5, and the reaction gas is further cooled to 30-40°C; the temperature of the absorption water in the absorption tower is 2-3°C, and the mass flow ratio to acetonitrile is 12-15; the recovery tower top The reflux ratio is 3.0-4.0, and the temperature control range of the sensitive point in the stripping section is 60-70°C;
[0129] In the refining process, the reflux ratio at the top of the decyanation tower is 3.0-4.0, and the operating pressure is 0.06-0.08Mpa absolute pressure; the ratio of the reflux flow at the top of the dehydration tower to the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com