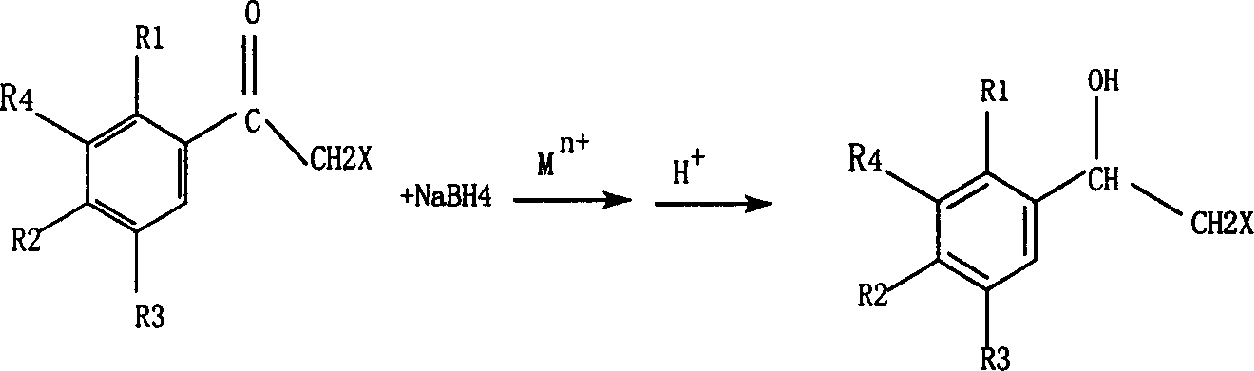
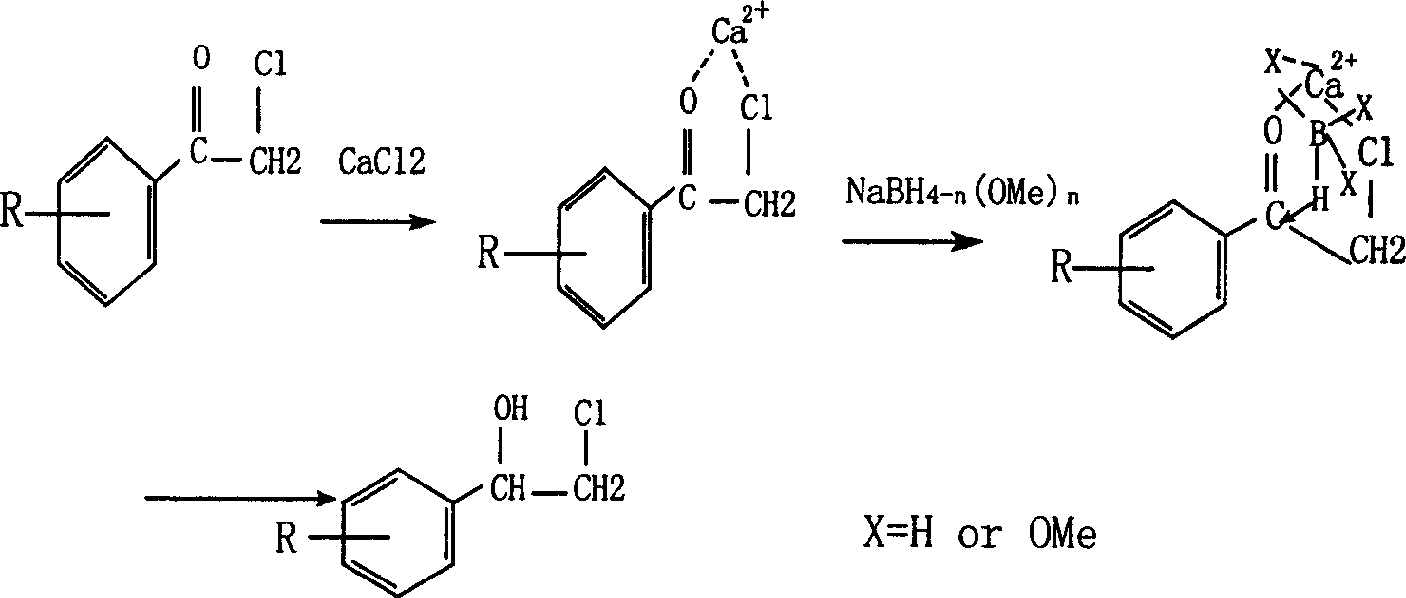
Process for preparing beta-halogen-alpha-phenyl ethyl alcohol compounds
A compound, phenylethyl alcohol technology, applied in the field of preparation of β-halogenated-α-phenylethyl alcohol compounds, can solve the problems of reduced reaction selectivity and yield, difficult separation and purification, low reaction efficiency, etc., reaching the reaction temperature range Wide, high yield, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Synthesis of 1-(2,4-dichlorophenyl)-2-chloro-ethanol
[0024] Add 0.2mol (22.2g) of calcium chloride, 0.1mol (22.35g) of 2,2',4'-trichloroacetophenone, and 90ml of methanol into a 250ml three-necked flask, and stir at 25°C for 0.5 hours. Dissolve 0.12mol (4.8g) of 96% sodium borohydride in a solution made of 10ml of methanol + 10ml of 1% aqueous sodium hydroxide solution. Under ice bath and stirring, sodium borohydride solution was added dropwise. After the dropwise addition, the stirring reaction was continued at room temperature for 20 minutes, and a white solid was produced as the reaction progressed. Add 50ml (1+2) of hydrochloric acid solution to decompose. Most of the methanol was distilled off under reduced pressure. Cool to room temperature, separate the organic layer, extract the aqueous phase with ether (20ml×2), combine the extract into the organic phase, and dry over anhydrous magnesium sulfate. Ethyl ether was distilled off to obtain 22 g ...
Embodiment 2
[0026] Embodiment 2: Synthesis of 1-(2,4-dichlorophenyl)-2-chloro-ethanol
[0027] 0.20mol (39.58g) manganese dichloride (MnCl 24H2O), 0.10mol (22.35g) 2,2', 4'-trichloroacetophenone, 100ml95% ethanol, join in the 250ml there-necked flask, and stir at 25°C for 0.5 hour. Dissolve 0.15mol (5.70g) of 96% sodium borohydride in 10ml of 1% aqueous sodium hydroxide solution, and slowly add the sodium borohydride solution dropwise at room temperature with stirring. After the dropwise addition, the stirring reaction was continued for 30 minutes under heating in a water bath at 70° C., and the following treatment was the same as in Example 1. 22 g of a yellow oily liquid product was obtained, and white crystals were precipitated upon standing. Analysis by gas chromatography showed that the product 1-(2,4-dichlorophenyl)-2-chloro-ethanol had a purity of 99.8%. 20 g of white needle-like crystals were obtained by recrystallization with 20 ml of petroleum ether, with a yield of 88.7%.
Embodiment 3
[0028] Embodiment 3: Synthesis of 1-(2,4-dichlorophenyl)-2-chloro-ethanol
[0029] Add 0.1mol zinc chloride (13.63g) or 0.1mol zinc nitrate (29.75g), 0.05mol (11.175g) 2,2',4'-trichloroacetophenone, 50ml methanol into a 250ml three-necked flask, Stir at 25°C for 0.5 hours. Dissolve 0.075mol (2.85g) of 96% sodium borohydride in 5ml of methanol + 8ml of 1% aqueous sodium hydroxide solution, and slowly add the sodium borohydride solution dropwise in an ice-water bath with stirring. After the dropwise addition, the stirring reaction was continued for 30 minutes under heating in a water bath at 50°C. The following treatment was the same as in Example 1. 12 g of a yellow oily liquid product was obtained, and white crystals were precipitated upon standing. Analysis by gas chromatography showed that the product 1-(2,4-dichlorophenyl)-2-chloro-ethanol had a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com