Liquid-phase synthesis of anode material for lithium ion secondary battery
A cathode material and a technology for secondary batteries, which are applied in the field of preparing cathode materials for lithium ion batteries, can solve the problems of uneven composition of synthetic materials, time-consuming, high calcination temperature, and achieve good industrial application prospects, shorten sintering time, and high charge-discharge capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
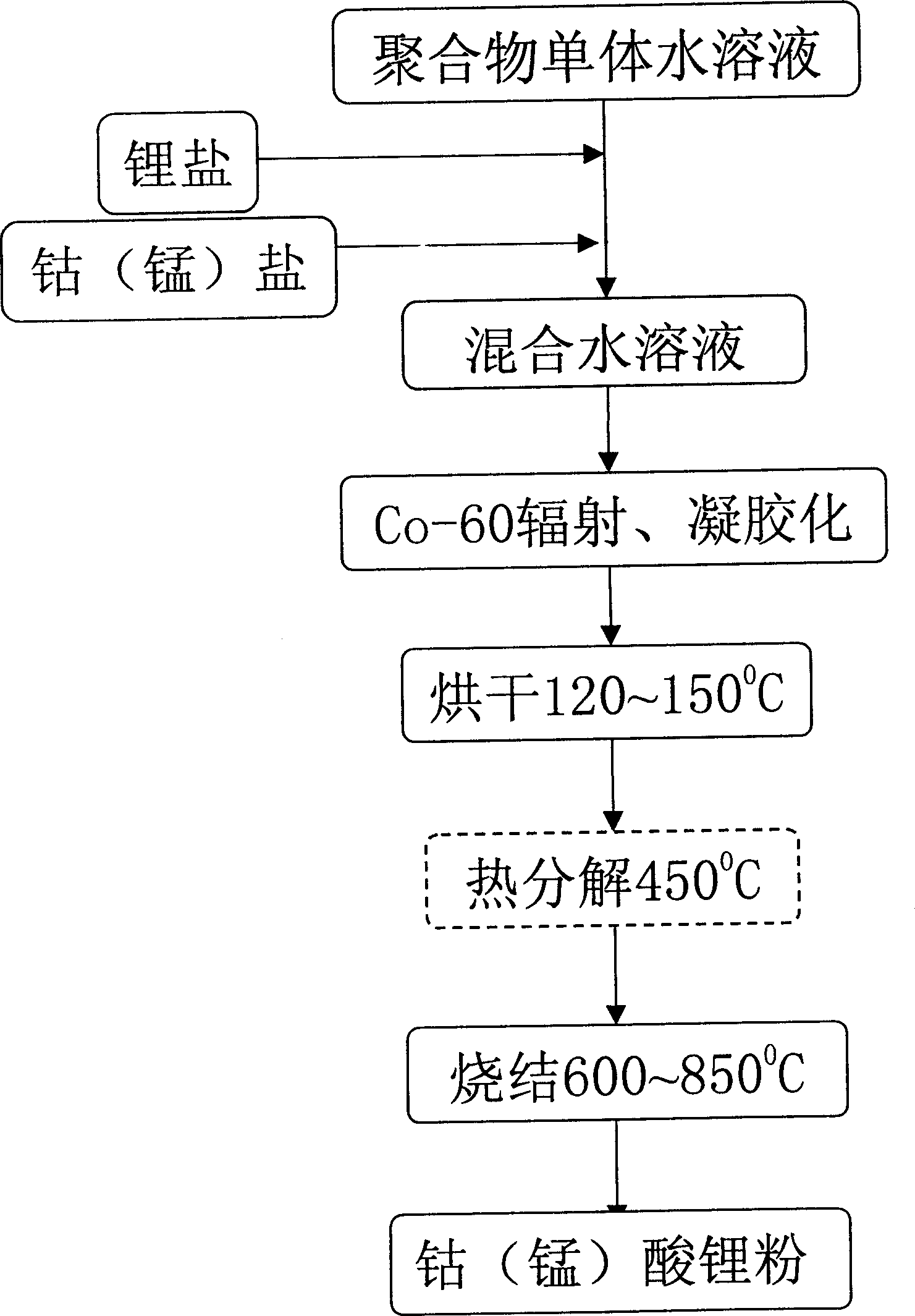
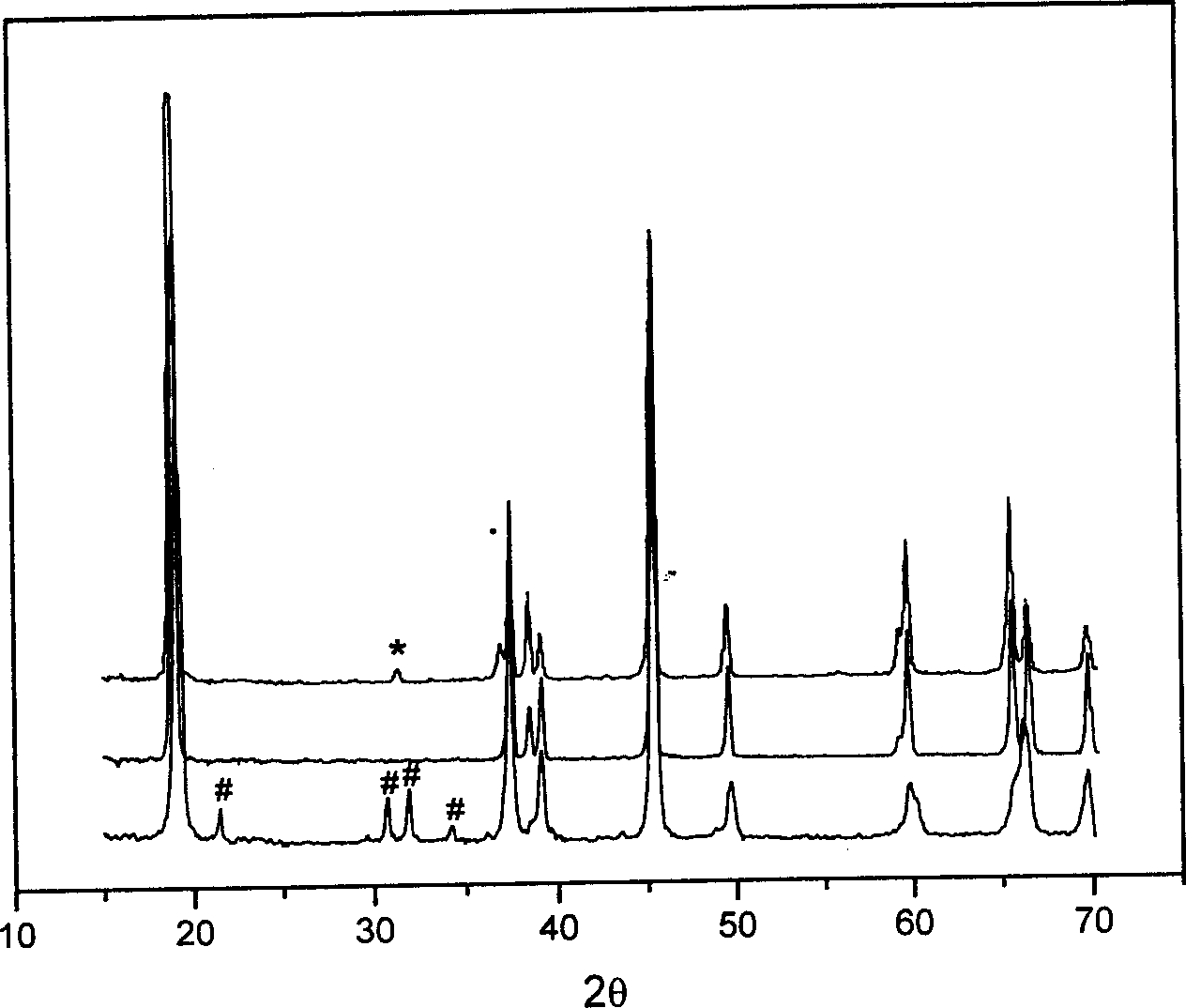
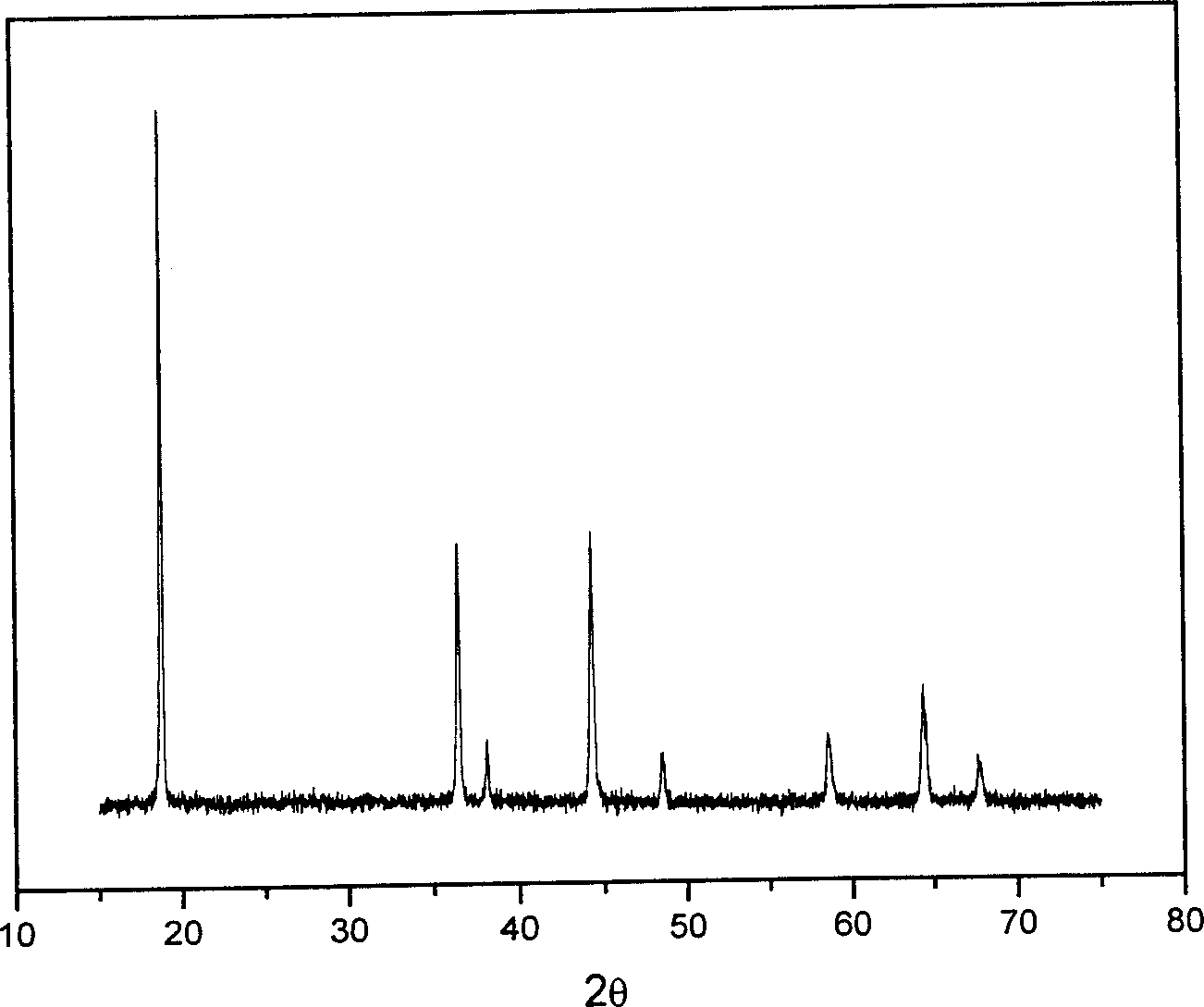
[0020] Take 2.8969g of LiNO respectively 3 and 11.6412g of Co(NO 3 ) 2 ·6H 2 O (that is, the molar ratio of Li:Co is 1.05:1), was stirred and dissolved in 10mL of water, and then 10mL of acrylic acid was added. According to the dose rate of 55Gy / min, the irradiation time is 90min), the purple polymer gel is finally obtained, the gel is dried at 100℃~150℃, then slowly heated to 450℃, thermally decomposed for 2 hours, and removed Organic matter in the gel, forming LiCoO 2 Powder precursor. Then the LiCoO was sintered under air atmosphere 2 powder precursor, the sintering temperature is 800°C, and the sintering time is 10 hours, and finally LiCoO 2 powder.
[0021] LiCoO prepared with Example 1 2 The powder is used as a half-battery on a lithium sheet, and the electrolyte used is 1mol / L LiPF 6 / (EC+DEC) (where EC is ethylene carbonate, DEC is diethyl carbonate, the volume ratio of the two is 1:1), the test voltage range is 2.7V ~ 4.2V, and the current density is 0.67mA / cm...
Embodiment 2
[0023] Take 1.8099g of LiNO respectively 3 and 7.2758g of Co(NO 3 ) 2 ·6H 2 O (that is, the molar ratio of Li:Co is 1.05:1), stirred and dissolved in 50mL of water, then added 50mL of acrylic acid, after mixing evenly, used Co-60 as a radiation source to carry out radiation polymerization, and the radiation dose was 8000Gy ( According to the dose rate of 80Gy / min and the irradiation time of 100min), the polymer gel was obtained, and the gel was dried at 100°C to 150°C, and then slowly heated to 450°C to decompose the organic matter in the gel to form LiCoO 2 Powder precursor. Then the LiCoO was sintered under air atmosphere 2 Powder precursor, the sintering temperature is 800°C, and the sintering time is 10 hours.
Embodiment 3
[0025] Take 1.8099g of LiNO respectively 3 and 7.2758g of Co(NO 3 ) 2 ·6H 2 O (that is, the molar ratio of Li:Co is 1.05:1), stirred and dissolved in 12.5mL of water, then added 12.5mL of acrylic acid, after mixing evenly, used Co-60 as the radiation source to carry out radiation polymerization, and the radiation dose was 2000Gy (The irradiation dose rate is 50Gy / min, and the irradiation time is 40min). Finally, the polymer gel is dried at 100°C to 150°C, and then slowly heated to 450°C to decompose the organic matter in the gel. , forming LiCoO 2 Powder precursor. Then the LiCoO was sintered under air atmosphere 2 Powder precursor, sintered at 800°C for 10 hours to obtain LiCoO 2 powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com