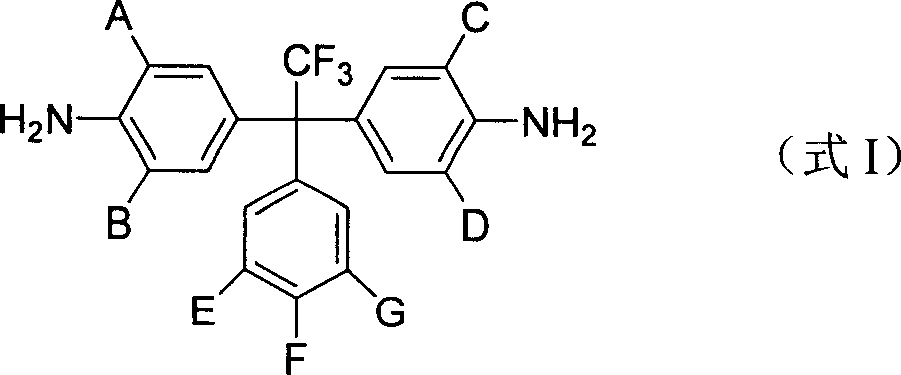
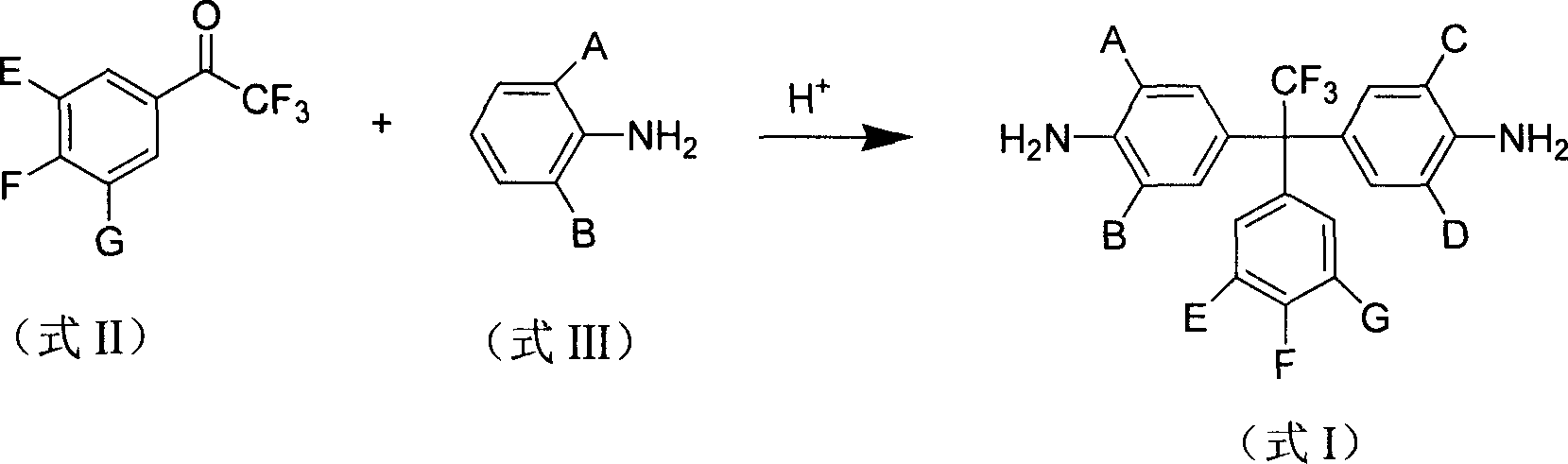
Intrinsical fluorinated photosensitive polyimide resin and its preparation method
A photosensitive polyimide resin, an intrinsic type technology, is applied in the field of intrinsic type fluorinated photosensitive polyimide resin and its preparation, and achieves the effects of low water absorption, tolerant photolithography conditions and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
[0034] Example 1, 1,1-bis(4'-amino-3',5'-bis(methyl)phenyl)-1-(3',5'-bis(trifluoromethyl)phenyl)- Synthesis of 2,2,2-trifluoroethane
[0035] 65 grams of 2,6-dimethylaniline, 35 grams of 2,6-dimethylaniline hydrochloride and 35 grams of 3',5'-bis(trifluoromethyl)-2,2,2 trifluoroacetophenone grams were mixed well in the reaction vial. The mixture was heated to 130°C for 1 hour until the starting material was completely dissolved. The reaction mass was then heated to reflux at 180°C for 10 hours. The temperature of the reaction system was cooled to room temperature, and 100 g of 20% sodium hydroxide solution was dropped into the reaction solution. Then the solution was distilled with steam for 4 hours, the insoluble matter was filtered and washed with water, then dried and recrystallized with ethanol to obtain a pure product with a reaction yield of 49%.
[0036] Product melting point: 151°C;
[0037] Infrared (KBr, cm -1 ): 3494 (N-H absorption peak), 3403 (N-H amino abso...
Embodiment 2、1
[0042] Example 2, 1,1-bis(4'-amino-3',5'-di(methyl)phenyl)-1-(4'-trifluoromethylphenyl)-2,2,2- Synthesis of trifluoroethane
[0043] 100 grams of 2,6-dimethylaniline, 40 grams of 2,6-dimethylaniline hydrochloride and 50 grams of 4'-trifluoromethyl-2,2,2 trifluoroacetophenone are mixed in a reaction flask uniform. The mixture was heated to 120°C for 1.5 hours until the starting material was completely dissolved. The reaction mass was then heated to reflux at 170°C for 8 hours. The temperature of the reaction system was cooled to room temperature, and 100 g of 20% potassium hydroxide solution was dropped into the reaction solution. Then the solution was distilled with steam for 4 hours, the insoluble matter was filtered and washed with water, then dried and recrystallized with ethanol to obtain a pure product.
[0044] Product melting point: 162°C;
[0045] NMR-H spectroscopy (300 MHz, DMSO-d 6 , δ): 2.3(S; 12H), 4.8(S; 4H), 6.5(S; 4H), 7.0(D; 2H), 7.4(D; 2H);
[0046] NM...
Embodiment 3、1
[0048] Example 3, 1,1-bis(4'-amino-3',5'-di(methyl)phenyl)-1-(3'-trifluoromethylphenyl)-2,2,2- Synthesis of trifluoroethane
[0049] Mix 80 grams of 2,6-dimethylaniline, 25 grams of 2,6-dimethylaniline hydrochloride and 45 grams of 3'-trifluoromethyl-2,2,2 trifluoroacetophenone in a reaction flask uniform. The mixture was heated to 120°C for 2 hours until the starting material was completely dissolved. The reaction mass was then heated to reflux at 170°C for 12 hours. The reaction system was cooled to room temperature, and 100 g of 20% ammonia solution was dropped into the reaction solution. Then distill the solution with water steam for 4 hours, filter the insoluble matter and wash with water, after drying, use petroleum ether-ethyl acetate (volume ratio 1:1) mixture as eluent, and obtain pure product by silica gel column chromatography .
[0050] Product melting point: 149°C;
[0051] NMR-H spectroscopy (300 MHz, DMSO-d 6 , δ): 2.4(S; 12H), 4.0(S; 4H), 6.6(S; 4H), 7.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com