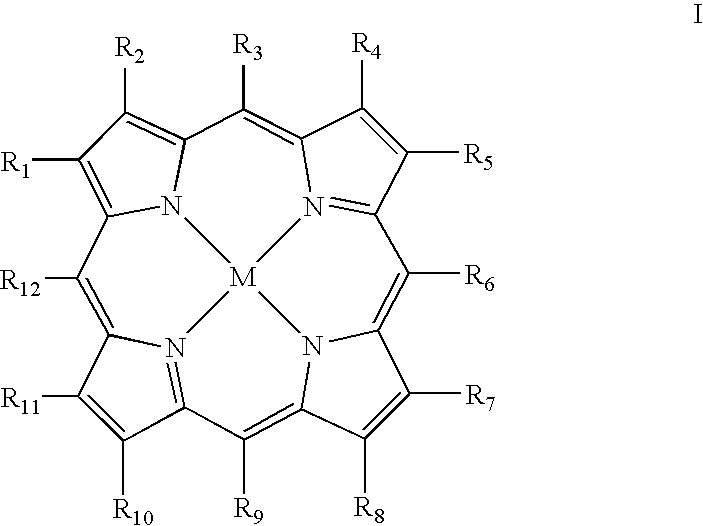
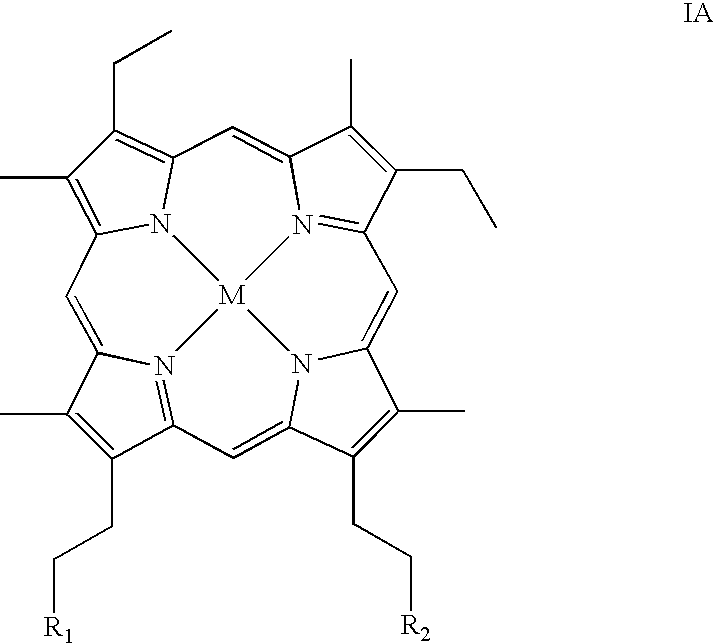
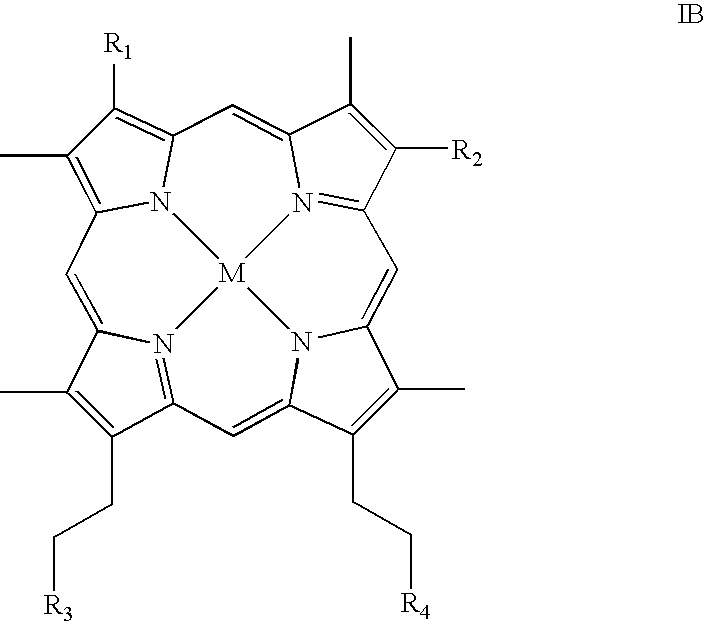
Metallotetrapyrrolic photosensitizing agents for use in photodynamic therapy
a technology of photosensitizing agent and metal tetrapyrrol, which is applied in the field of metalotetrapyrrolic photosensitizing agent for use in photodynamic therapy, can solve the problems of ischemic heart disease, reduced blood flow, and increased blood pressure, and the biochemical factors that result in the preferential uptake of some photosensitizers in certain tissue types compared to others,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Gallium Chloride Mesoporphyrin Dimethyl Ester
[0279] Mesoporphyrin dimethyl ester (610 mg) was dissolved in acetic acid (75 mL) and Gallium acetyl acetonate added (700 mg). The solution was refluxed for 1 hr after which time a UV visible analysis of the molecule showed the metallation to be complete. The solvent was removed by rotary evaporation and the residue dissolved in dichloromethane (100 mL). The dichloromethane layer was washed repeatedly with 1N HCl and the organic layer collected and evaporated. The crude reaction mixture was chromatographed on silica (7.5% methanol / dichloromethane) and the major pink fraction collected and evaporated. The compound was redissolved in dichloromethane (100 mL), the organic layer was washed repeatedly with 1N HCl, dried over sodium sulfate and evaporated to ˜10 mL. Hexane was added (7 mL) and the dichloromethane was removed by rotary evaporation. The precipitated solid was collected by filtration and dried. Yield of the title compound=650 mg....
example 2
Gallium Chloride Mesoporphyrin Diethyl Ester
[0280] Mesoporphyrin dimethyl ester (200 mg) was refluxed in 5% sulfuric acid in ethanol (25 ml) for 6 hrs. The reaction was cooled to room temperature, diluted with water (100 ml) and solution neutralized with sodium bicarbonate. The solid was filtered, dried and crystallized from dichloromethane and ethanol. Yield of mesoporphyrin diethyl ester=180 mg. This was then metallated as described in example 1. Yield of the title compound=190 mg.
example 3
Gallium Chloride Mesoporphyrin Dipropyl Ester
[0281] Mesoporphyrin dimethyl ester (150 mg) was refluxed in 2% sulfuric acid in propanol (30 ml) for 6 hrs. The reaction was cooled to room temperature, diluted with water (100 ml) and solution neutralized with sodium bicarbonate. The solid was filtered and dried. Yield of mesoporphyrin dipropyl ester=180 mg. This was then metallated as described in example 1. Yield of the title compound=190 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| atomic numbers | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com