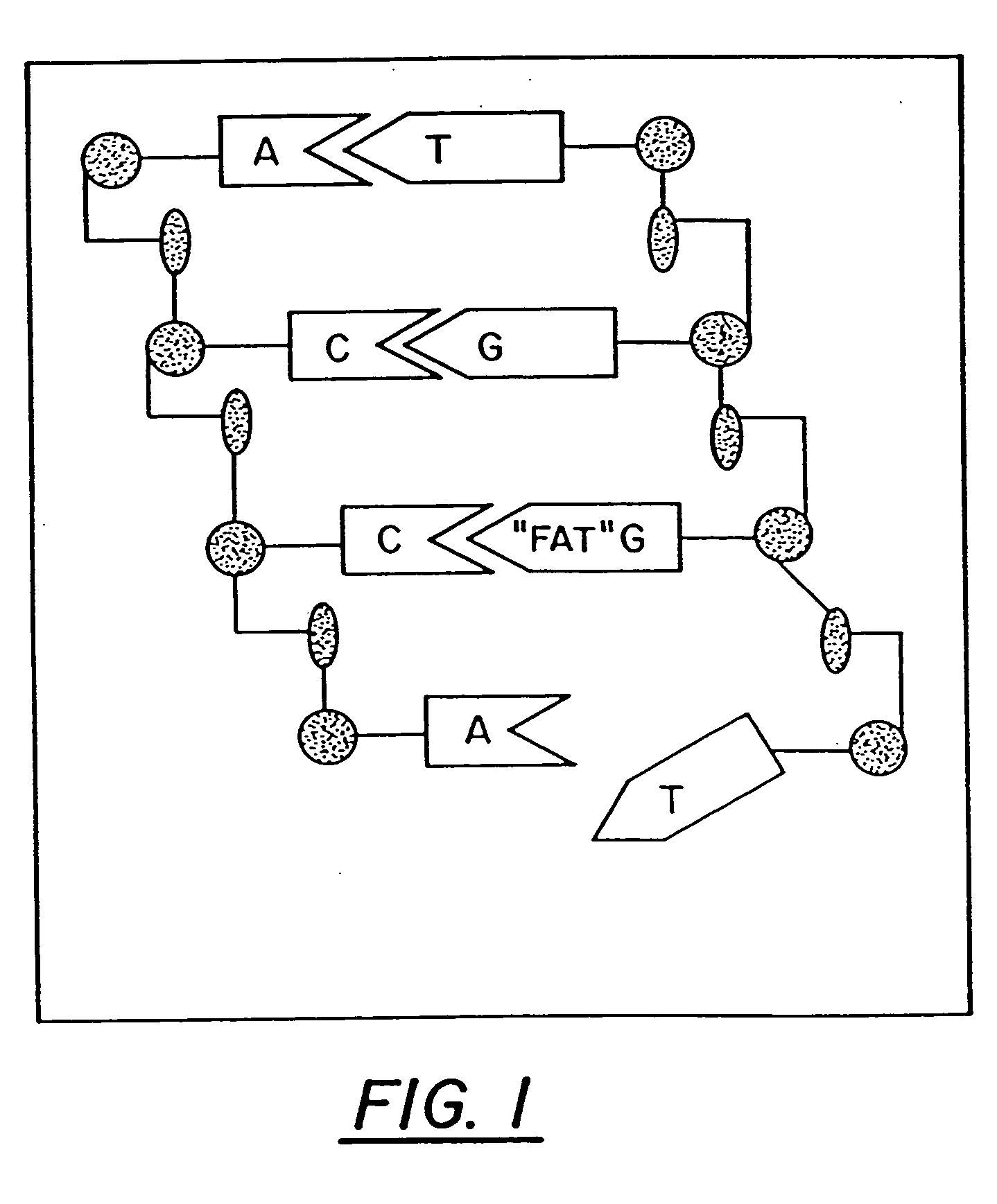
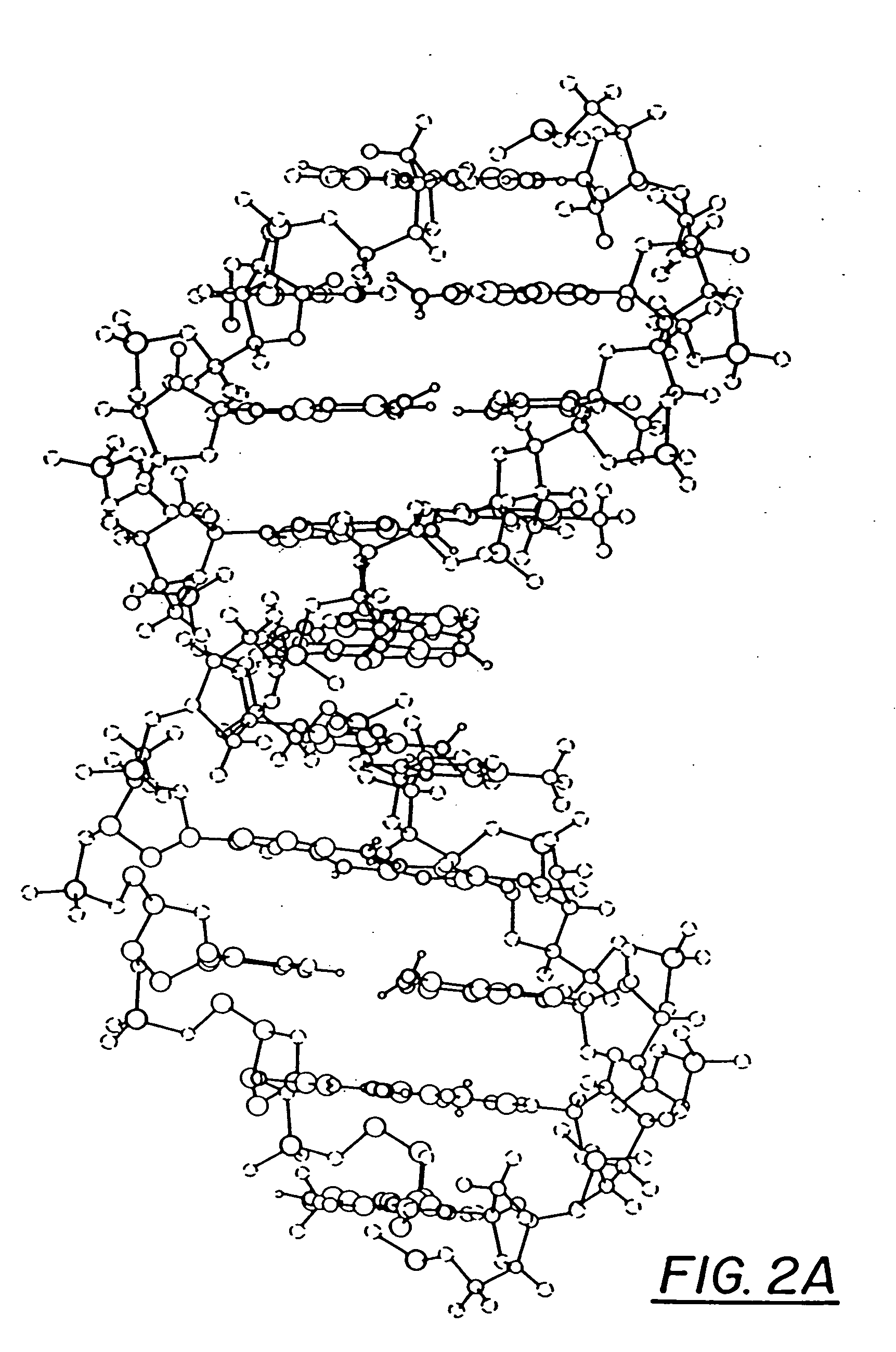
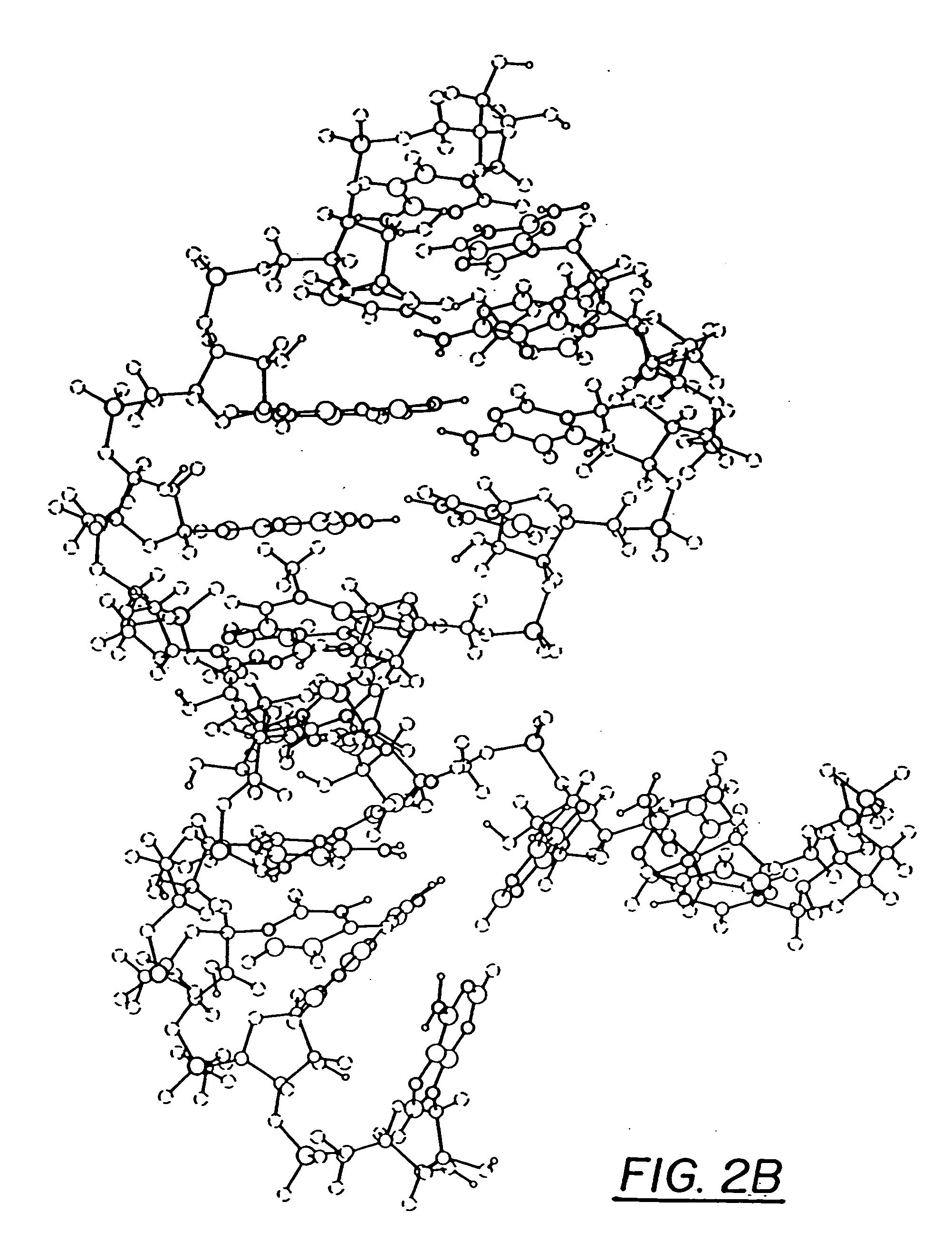
Ring-expanded nucleosides and nucleotides
a nucleoside and ring-expanded technology, applied in the field of ring-expanded nucleosides and nucleotides, can solve the problems of insufficient approach alone to stop the epidemic, inability to prove effective therapeutically, and inability to meet the specificity and/or toxicity of many otherwise promising antiviral agents, so as to reduce the risk of toxicities, enhance the therapeutic effect, and increase the therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
[0178] The present invention can be illustrated by the use of the following non-limiting examples:
example 1
4,8-Diamino-6-imino-1H-imidazo[4,5-e][1,3]diazepine
Formula I(B), where U, Y, W, K=C; X, Z, J, L=N; R1, R5=NH2; R3=NH; R2, R4, R6=None; and R7, R8=H
Method A: By Condensation of 4,5-Dicyanoimidazole with Guanidine
[0179] Guanidine was liberated from guanidine hydrochloride (1.15 g, 12 mmol) by addition of a freshly prepared solution of sodium methoxide in methanol from sodium (0.28 g, 12 mmol), and stirring with ice-water cooling for 30 minutes. The precipitated sodium chloride was filtered off, and the filtrate was added to the solution of 4,5-dicyanoimidazole (1.18 g, 10 mmol) in methanol (25 mL). The reaction mixture was heated to reflux for 20 hours and cooled to room temperature. The solid separated was collected by filtration, and recrystallized from methanol. Yield 1.15 g (65%), mp>220° C.: 1H NMR (DMSO-d6) δ 7.75 (s, 2H, NH2, exchangeable with D2O); 7.65 (s, 2H, NH2, exchangeable with D2O), 7.53 (s, 1H, imidazole CH), 6.98 (s, 2H, two NH, exchangeable with D2O);
[0180]13C NM...
example 2
6-Imino-1H-imidazo[4,5-e][1,3]diazepine-4,8-dione
Formula I(A), where U, Y, W, K=C; X, Z, J, L=N; R1, R5=O; R3=NH; R2, R4, R6, R7=H; R8=None
[0184] (a) 4,5-Imidazolediformyl Chloride: 4,5-imidazoledicarboxylic acid (2.0 g, 12.8 mmol) was placed in a flame-dried three-necked, round-bottomed flask, fitted with a CaCl2 guard tube. Thionyl chloride (10 mL, 0.137 mol) was introduced through a serum cap, and the reaction mixture was heated at 50° C. with continuous stirring for 24 hours. The reddish-yellow reaction mixture was rotary evaporated to dryness under anhydrous conditions, and the residue was coevaporated to dryness with dry toluene (3×10 mL). The resultant residue was employed for the next step given below without further purification.
[0185] (b) 6-Imino-1H-imidazo[4,5-e][1,3]diazepine-4,8-dione: Guanidine was liberated from guanidine hydrochloride (0.955 g, 10 mmol) by addition of a freshly prepared solution of sodium methoxide in methanol from sodium (0.23 g, 10 mmol), and st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com