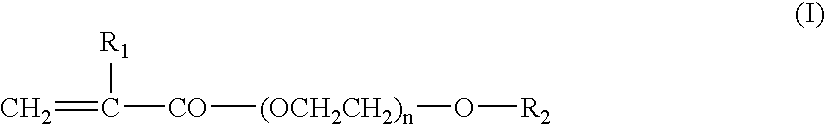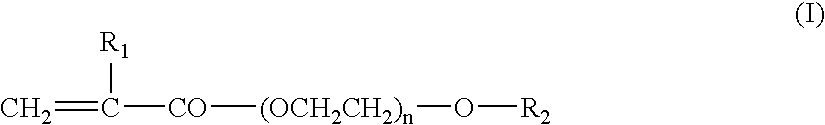Binder Resin Composition for Nonaqueous Electrolyte Energy Device Electrode, Nonaqueous Electrolyte Energy Device Electrode, and Nonaqueous Electrolyte Energy Device
a technology of nonaqueous electrolyte energy device electrode and binder resin, which is applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of impede the effort to raise the capacity of lithium batteries, poor adhesion of pvdf, and insufficient pvdf, etc., to achieve excellent adhesion, excellent resistance to swelling, and high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0057] A reaction solution was prepared by introducing, under a nitrogen blanket, 45.0 g acrylonitrile (nitrile group-containing monomer, from Wako Pure Chemical Industries, Ltd.), 5.0 g lauryl acrylate (Aldrich, monomer with Formula (II), 0.0232 mol per 1 mol acrylonitrile), 1.175 mg potassium persulfate (polymerization initiator, from Wako Pure Chemical Industries, Ltd.), 135 mg α-methylstyrene diner (chain-transfer agent, from Wako Pure Chemical Industries, Ltd.), and 450 mL purified water (Wako Pure Chemical Industries, Ltd.) into a 1.0-liter separable flask equipped with a stirrer, thermometer, and reflux condenser. The reaction solution was vigorously stirred for 3 hours at 60° C. and 3 hours at 80° C. After cooling to room temperature, the reaction solution was suction-filtered and the precipitated resin was filtered off. The filtered-off resin was washed in sequence with 300 mL purified water (Wako Pure Chemical Industries, Ltd.) and 300 mL acetone (Wako Pure Chemical Indust...
example 8
[0061] Composite slurries were prepared by mixing amorphous carbon with an average particle size of 20 μm and the binder resin composition-containing varnish prepared in Example 1 (containing 10 mass % binder resin composition) in the range from 99.0 mass %: 10.0 mass % (1.0 mass % as resin fraction) to 95.0 mass %: 50.0 mass % (5.0 mass % as resin fraction) and adding NMP for viscosity adjustment. These were uniformly coated on 10 μm-thick copper foil (current collector), and a sheet-shaped electrode was prepared by then drying for 1 hour in a convection dryer set at 80° C. Seven sheet-shaped electrodes having different resin fractions were prepared in this manner. Each sheet-shaped electrode was pressed with a roll press to prepare an electrode having a composite density of 1.5 g / cm3. The presence / absence of laminate debonding was visually inspected at this point in order to investigate the relationship to the resin fraction (mass %). It is presumed that the absence of laminate de...
example 15
[0064] Lithium manganate with an average particle size of 10 μm, carbon powder with an average particle size of 3 μm, and the binder resin composition-containing varnish obtained in Example 1 (binder resin composition content=10 mass %) were mixed in proportions of 87.0 mass %: 8.7: 43.0 mass % (4.3 mass % as the resin fraction), and a composite slurry was then prepared by the addition of NMP for viscosity adjustment. This slurry was coated and dried onto one side of 20 μm-thick aluminum foil. The composite is coated at the rate of 30 mg / cm2. This was followed by rolling with a roll press to give a composite density of 2.6 g / cm3; cutting into a circle with a diameter of 1.5 cm then gave a sheet-shaped positive electrode. The positive electrode was thereafter obtained by vacuum drying for 16 hours at 120° C. in order to remove residual solvent and adsorbed water in the electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass-transition temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com