Composite electrodes for lithium ion battery and method of making
a lithium ion battery and composite electrode technology, applied in the direction of sustainable manufacturing/processing, cell components, secondary cell details, etc., can solve the problems of poor electronic conductivity, limited lithium ion from liquid and energy storage particles, limiting the performance and the lifetime of traditional lithium ion cells, etc., to reduce internal porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
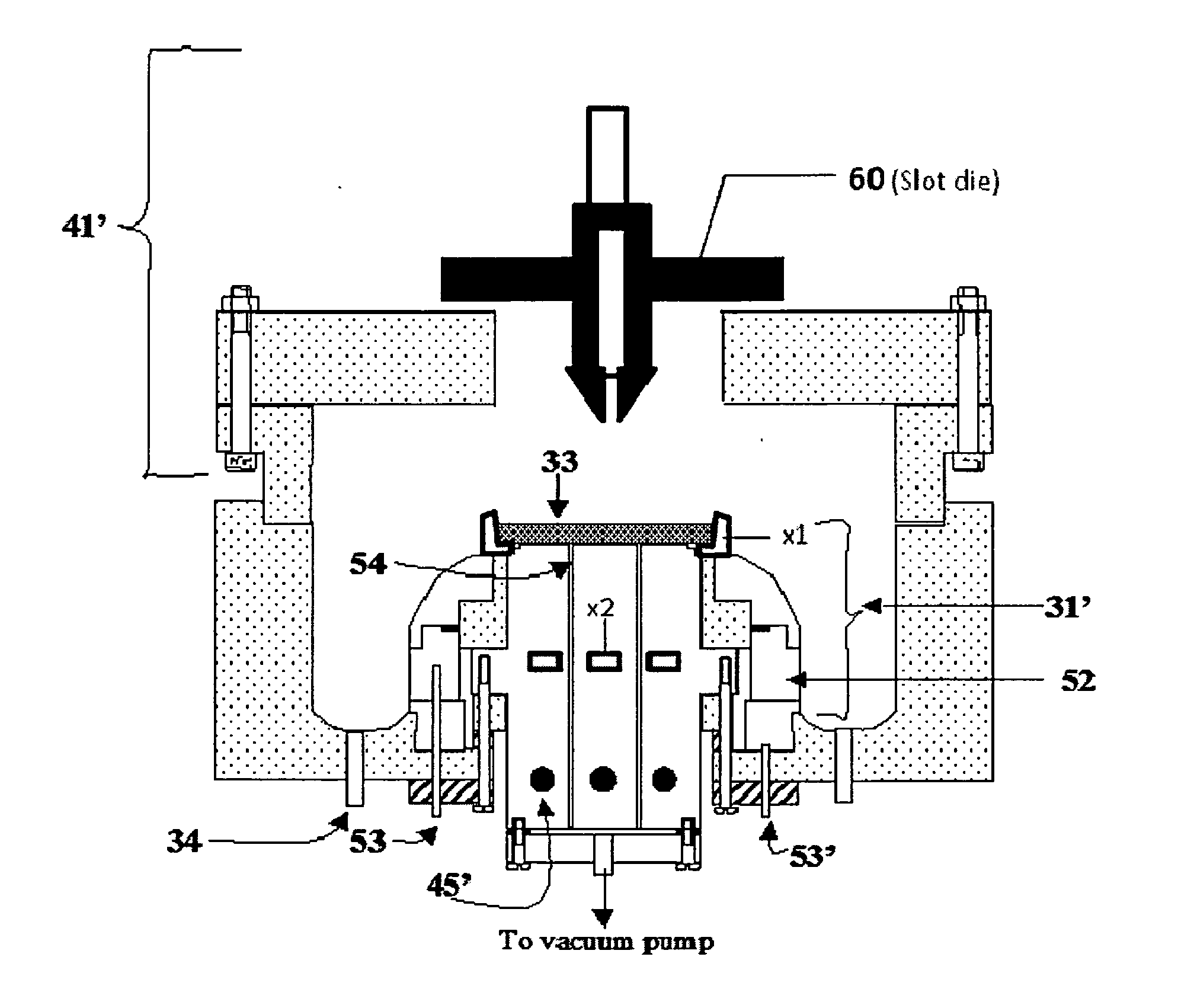
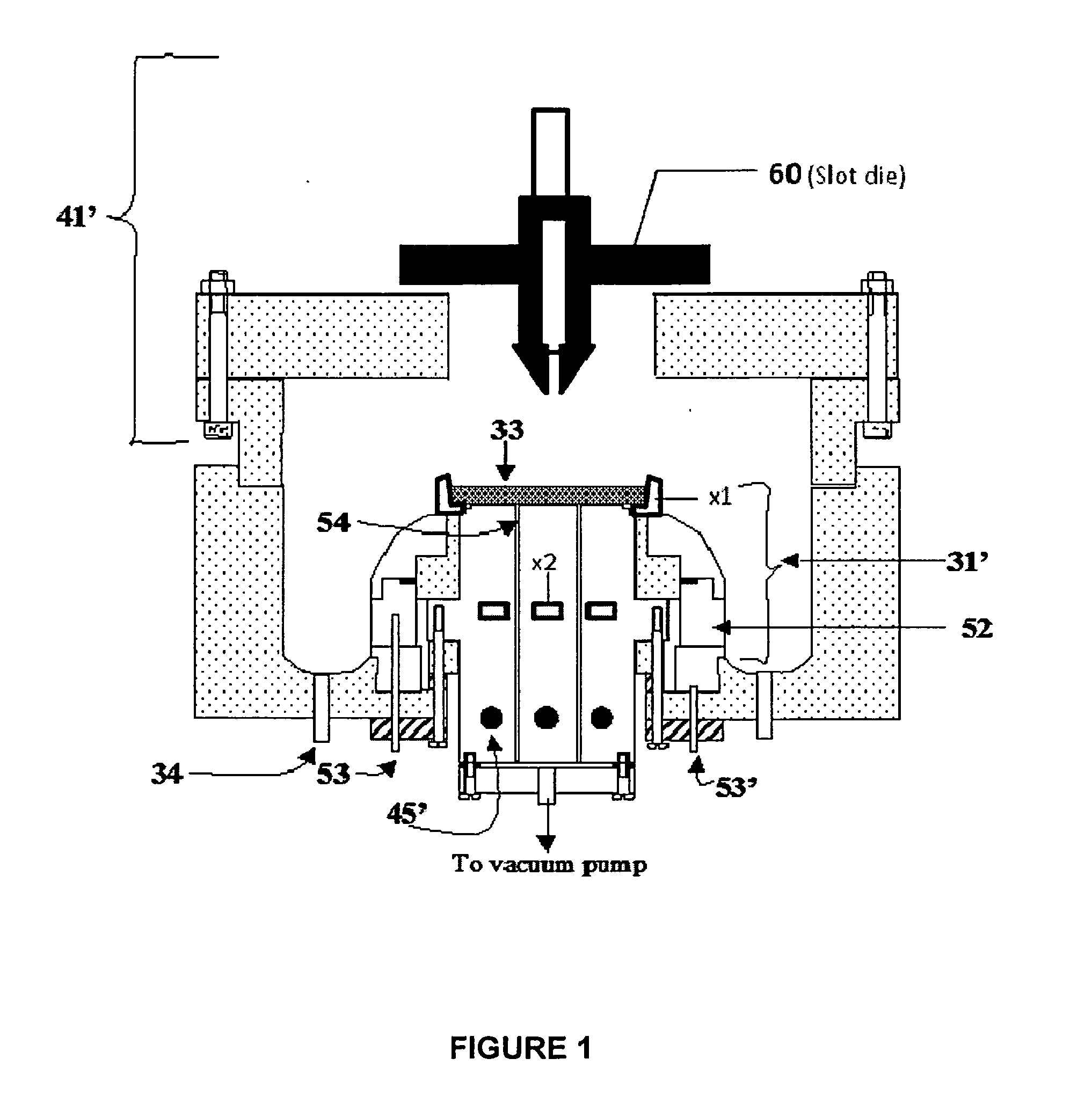
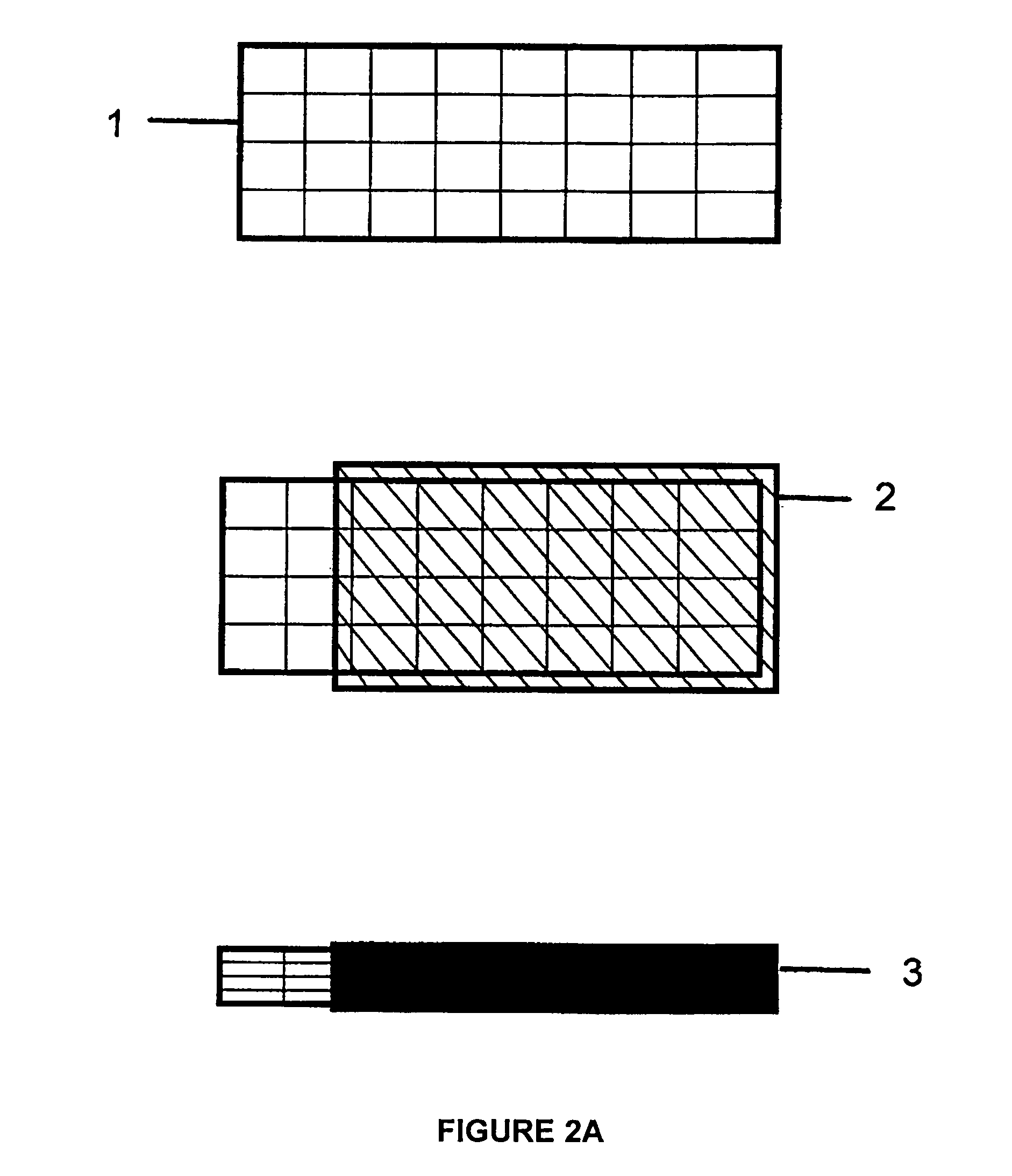
[0076]To form a LiCoO2:Al composite cathode, 9.0 g cobalt nitrate, 3 g urea, 1.0 g Al(NO3)3, and 3.0 g Li(NO3) were dissolved in 50 ml of de-ionized water and heated until the CoAlLi[complex]O nuclei is formed and the hot solution is 20 ml. 5 ml of 1M citric acid was then added. This was followed by 1 ml of 40 wt. % lithium polysilicate in deionized water. The mixture was then sonicated to form a gel. Then, 0.3 g of Li1.3Al0.3Ti1.7(PO4)3 and 0.3 g of TiOx nanoparticles were added for improved ionic conductivity and electronic conductivity respectively. The gel was then resonicated to homogenize the gel. The GELSPEED process was then used to populate a 3″×3″ Ni foam substrate 1 heated at 150° C. The coated foam 2 was cured at 250° C. for about 5 minutes. Coating and curing were repeated 2 more times. Additional curing was done in a box furnace at 300° C. for 10 minutes. This was followed by calendaring under a 100 ton press to compact and densify the self supporting composite LiCoO2:...
example 2
[0077]To form a CuS composite cathode, 5 g copper nitrate, 5 g thiourea, and 4 ml hydrazine monohydrate were dissolved in 50 ml de-ionized water and heated until the Cu[complex]S nuclei was formed and the hot solution was 20 ml. 4 ml of 1M acetic acid was then added. This was followed by 1 ml of 40 wt. % lithium polysilicate in deionized water. The mixture was then sonicated to form a gel. Then, 0.3 g Li1.3Al0.3Ti1.7(PO4)3 and 0.3 g TiOx nanoparticles were added for improved ionic conductivity and electronic conductivity respectively. The gel was then resonicated to homogenize the gel. The GELSPEED process was then used to populate a 3″×3″ Ni foam substrate heated at 150° C. The coated foam was cured at 200° C. for about 5 minutes. Coating and curing were repeated 2 more times. Additional curing was done in the tube furnace at 300° C. for 10 minutes in sulfur ambient. This was followed by calendaring under a 100 ton press to compact and densify the self supporting composite CuS cath...
example 3
[0078]To prepare a SnO composite anode, 5 g tin ethoxide, 0.4 g urea, 0.5 g Al(NO3)3, and 0.3 g Li(NO3) were dissolved in 50 ml of de-ionized water and heated until the SnAlLi[complex]O nuclei was formed and the hot solution is 20 ml. 4 ml of 1M acetic acid was then added. This was followed by 1 ml of 40 wt. % lithium polysilicate in deionized water. The mixture was then sonicated to form a gel. Then, 0.3 g Li1.3Al0.3Ti1.7(PO4)3 and 0.3 g TiOx nanoparticles were added for improved ionic conductivity and electronic conductivity respectively. The gel was then resonicated to homogenize the gel. The GELSPEED process was then used to populate a 3″×3″ Ni foam substrate heated at 150° C. The coated foam was cured at 250° C. for about 5 minutes. Coating and curing were repeated 2 more times. Additional curing was done in a box furnace at 300° C. for 10 minutes. This was followed by calendaring under a 100 ton press to compact and densify the self supporting composite SnO anode. The formed s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com