General Mass Spectrometry Assay Using Continuously Eluting Co-Fractionating Reporters of Mass Spectrometry Detection Efficiency
a mass spectrometry and reporter technology, applied in the field of mass spectrometry, can solve the problems of ion suppression or enhancement, adversely affecting the ability to assay or measure chemical and biological analytes, and affecting the amount of charged analytes that reach the detector of the mass spectrometer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0149]An example of using a chemical reporter set to measure the absolute level of ranitidine in human plasma. An inventive set of chemical reporters of mass spectrometry detection efficiency were created based on their predicted ability to elute in a continuous peak to peak manner during a portion of an LC separation. The HPLC retention index (R.I.) of salbutamol (R.I. 225), 7-methylxanthine (R.I. 225), 4-aminoantipyrine (R.I. 226), famotidine (R.I. 228), DL-4-hydroxy-3-methoxymandelic acid (R.I. 230), and trans-2-phenylcyclopropylamine hydrochloride (R.I. 232) were previously described in Hill, D W, et al J Anal Tox, 1994; 18: 233-242. These chemical reporters were dissolved in 5% formic acid and mixed in said concentrations: salbutamol (2 nanograms / uL), 7-methylxanthine (12 nanograms / uL), 4-aminoantipyrine (10 nanograms / uL), famotidine (200 nanograms / uL), DL-4-hydroxy-3-methoxymandelic acid (18 nanograms / uL), trans-2-phenylcyclopropylamine hydrochloride (10 nanograms / uL). A 5 nan...
example 2
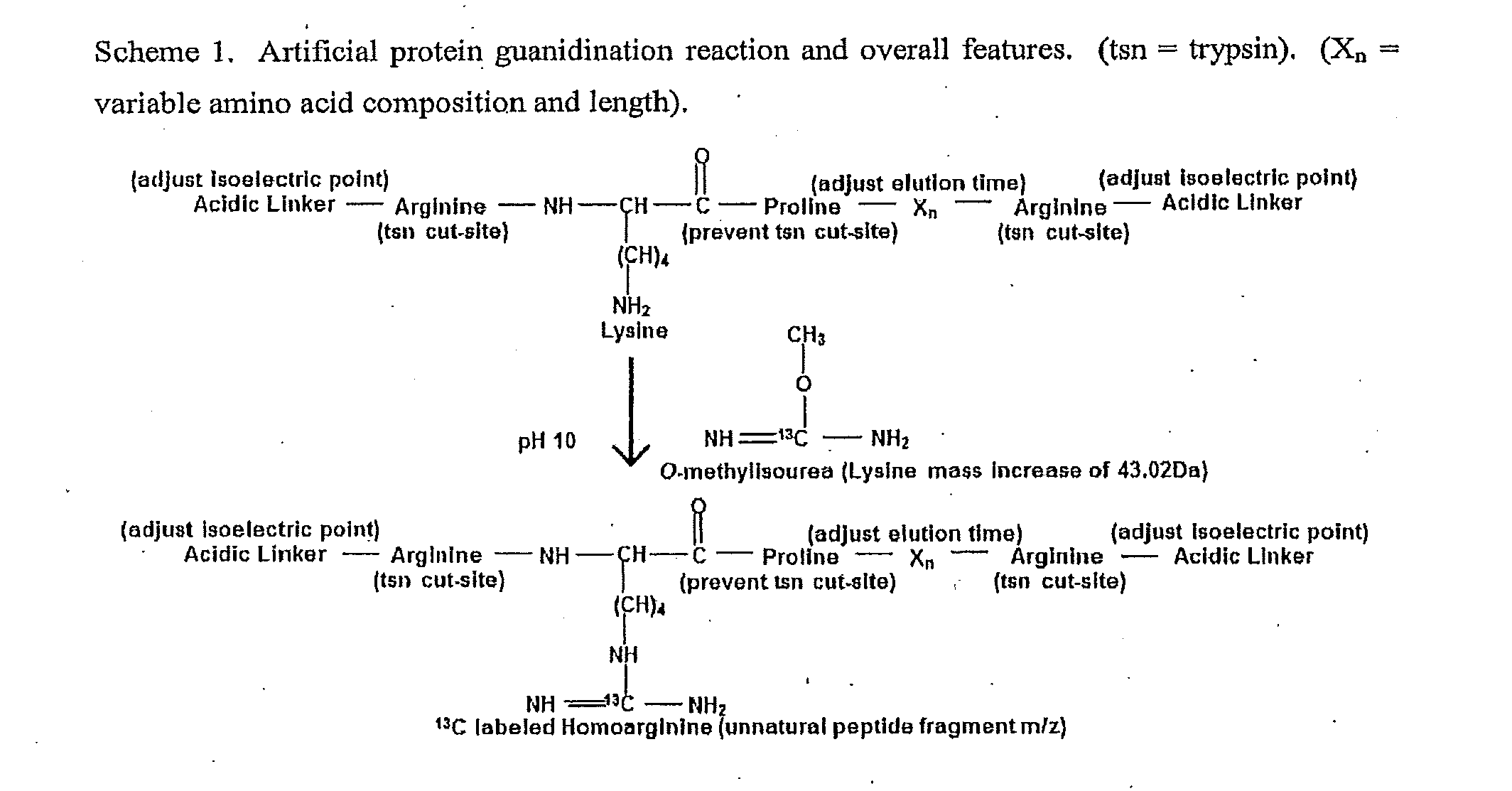
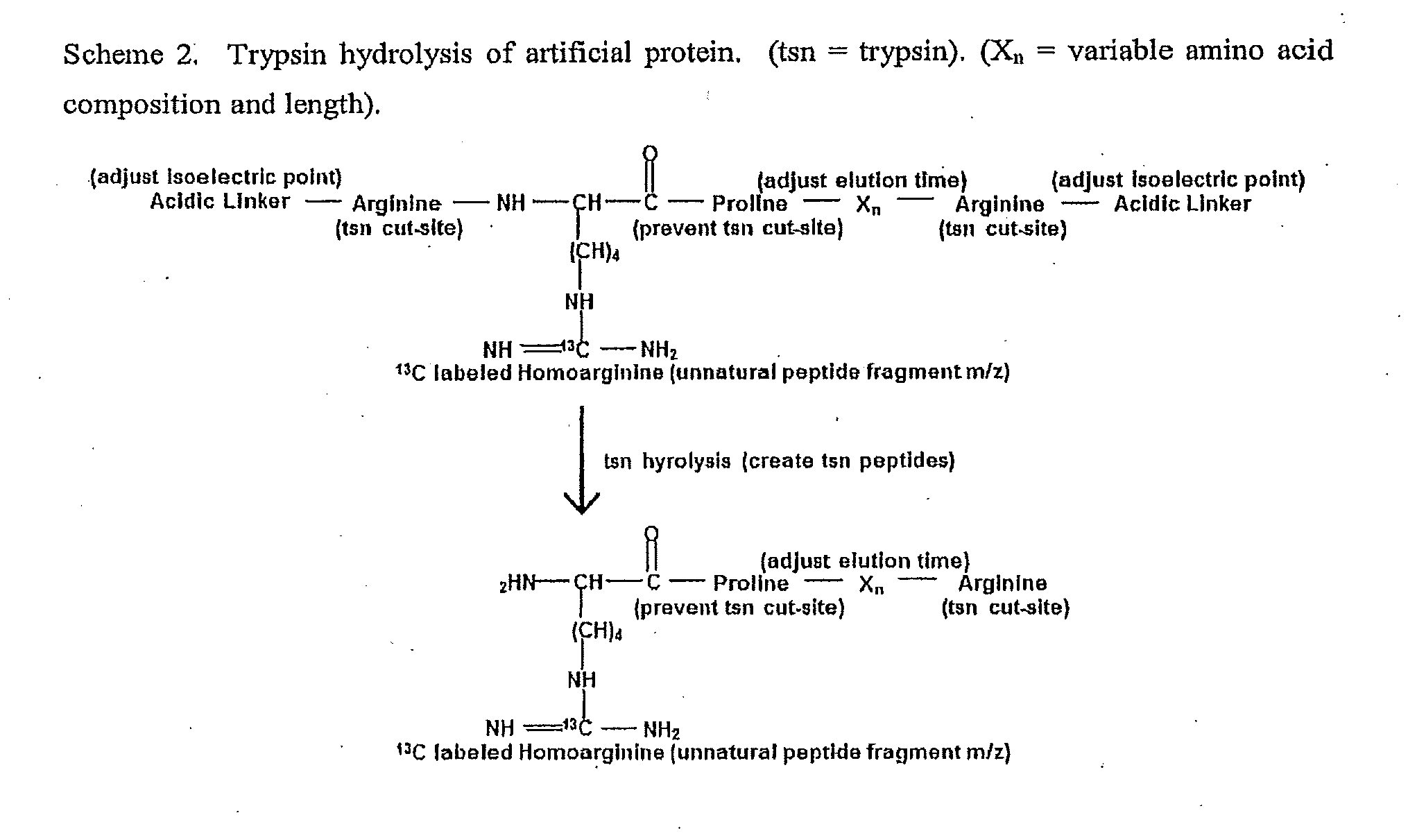
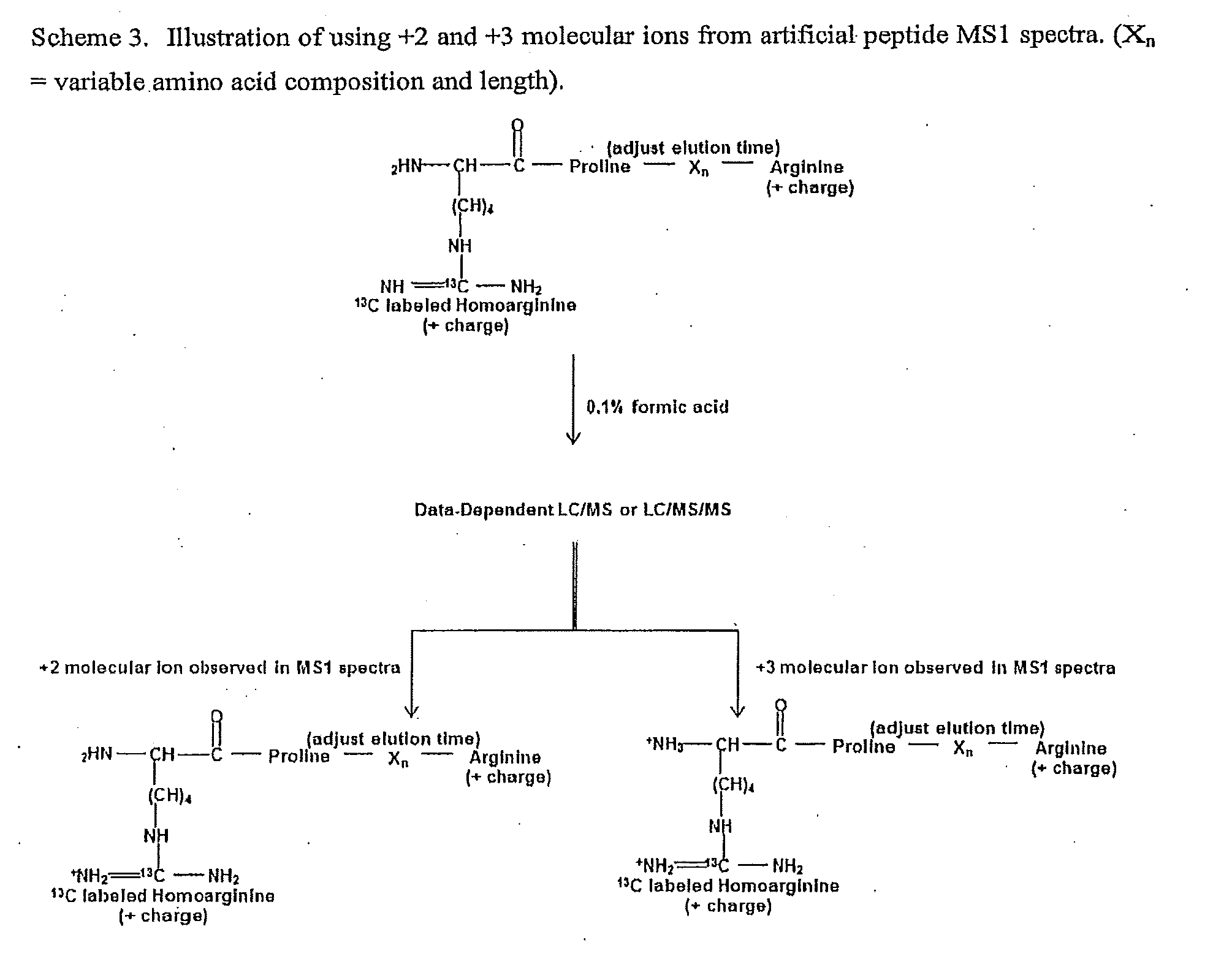
[0151]Design of artificial sequences: An in silico set of 20 artificial reporter peptides were created that were predicted by the Sequence Specific Retention Calculator Version 3.0 to elute in a peak to peak fashion during a fraction of the entire liquid chromatography separation with a 2% acetonitrile / minute linear gradient. These peptide sequences are shown in FIG. 7. and were checked to ensure that they do not exist in sequenced genomes by using the Prowl software program which searches sequenced genomes from the NCBI database (last updated on May 16, 2010) to ensure that the inventive peptides would not be generated by trypsin hydrolysis of proteins from the organisms of sequenced genomes. The peptides shown in FIG. 7. were assembled into an artificial protein and created by expression of the cDNA sequence shown in FIG. 8. This cDNA sequence was synthesized and subcloned into the NdeI and XhoI sites of the pET23b+ expression vector. A nucleotide sequence for protein expression o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com