Polymer-supported chelating agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the Polymer-Supported Chelating Agent
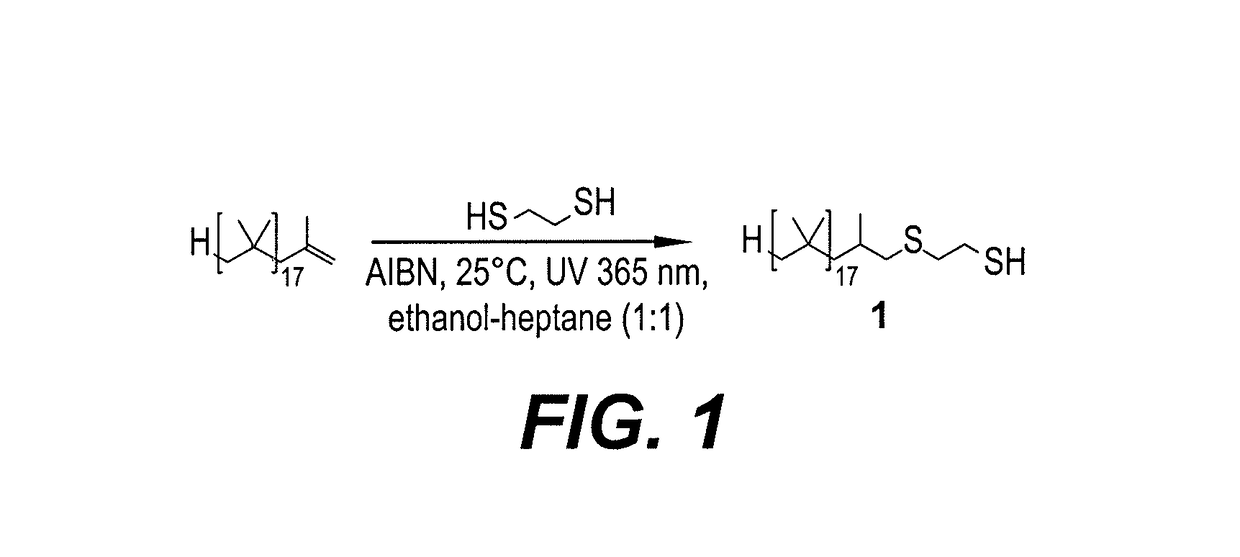
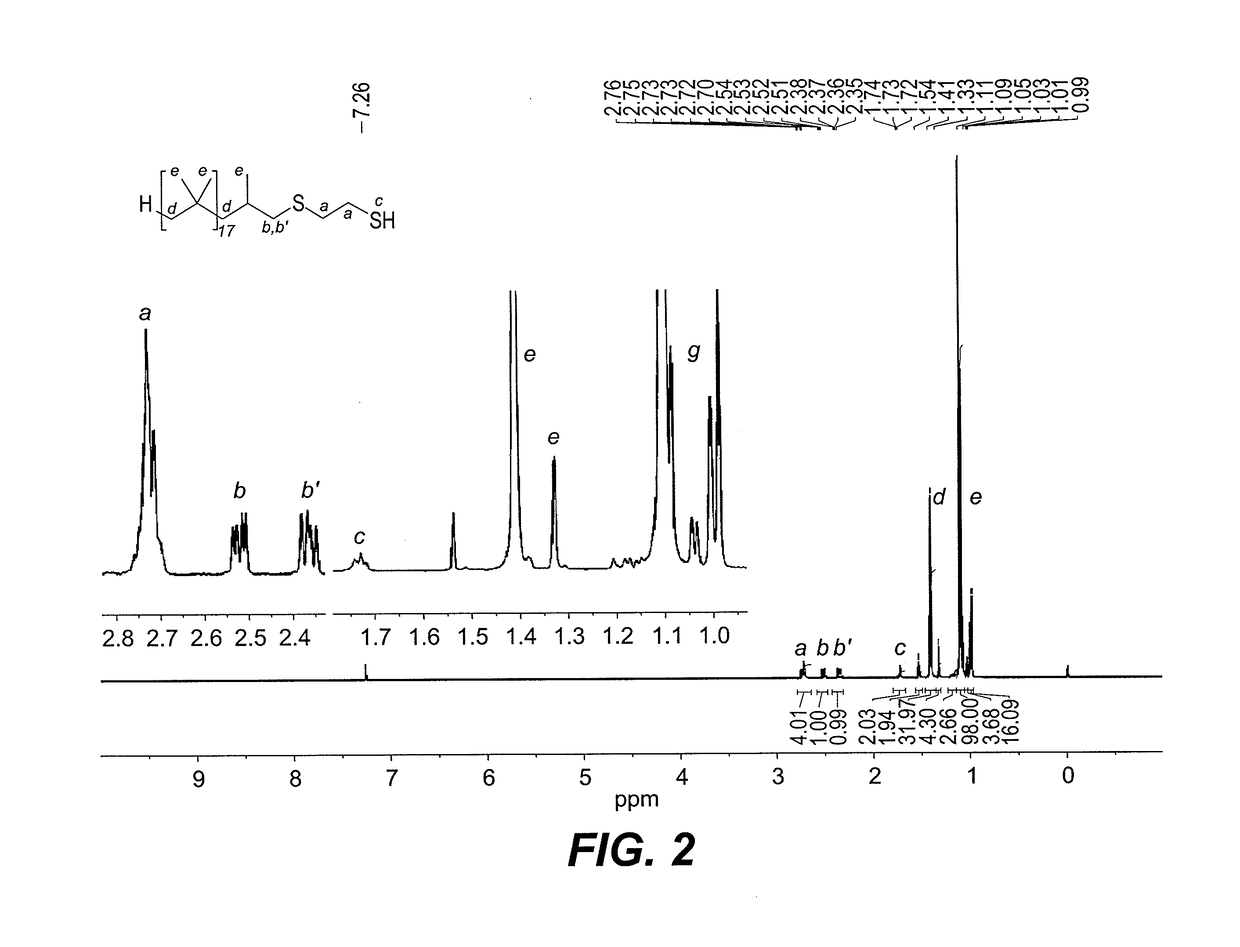
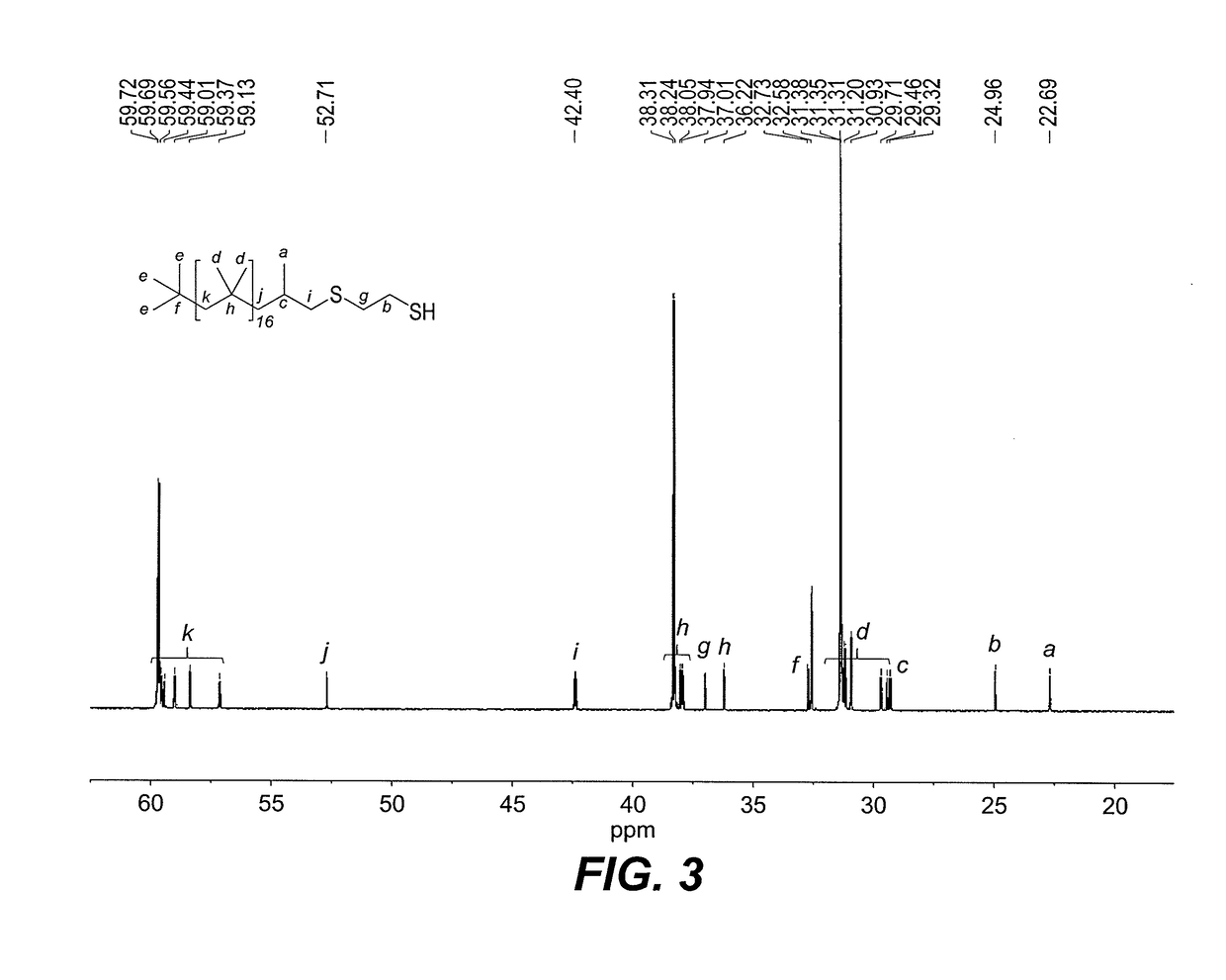
[0021]Dithiol-functionalized polybutadiene 1 was prepared via a green and simple single step radical thiol-ene “click” reaction between commercially available and inexpensive 1,2-ethanedithiol and alkene-terminated PIB Glissopal 1000 (DPn=18), as depicted in FIG. 1. The desired polymer 1 was obtained as clear viscous liquid in 92% yield and fully characterized by 1H (see FIG. 2) and 13C NMR spectroscopy (see FIG. 3). As is true for other functionalized PIB derivatives, NMR spectroscopy makes it easy to characterize the products, since the signals of the PIB backbone appear in the 1.00-1.50 ppm region, whereas signals of the functional terminus are observed downfield, from 1.50 to 3.00 ppm. In our first attempts, thermal initiation with either 0.1 eq. of di-tert-butyl peroxide (DTBP) or azobisisobutyronitrile (AIBN) at 70° C. led to complete transformation of the PIB alkene in 24 h. However, in both cases, the desired thioether-thiol ...
example 2
Proposed Mechanism of Action
[0023]The PIB-bound sequestrant we prepared contains two different binding sites—thioether and thiol. They have differing complexation activity and affinity to transition metals. 1H NMR spectroscopy titration of 1 with palladium acetate was used to understand better the complexation of 1 to Pd2+ (see FIG. 6). It led to a pronounced change in the chemical shift of the acetate protons from 2.00 ppm to 2.10 ppm that is characteristic for free acetic acid. Saturation was detected at equimolar [Pd2+]:[1] ratio by appearance of the signal of free palladium acetate complex. At this stage, signals of all three methylene groups adjacent to the sulfur atoms in 1 were shifted downfield with significant broadening, whereas the signal of the mercaptan hydrogen at 1.75 ppm disappeared, possibly due to proton exchange. These observations suggest chelation of Pd2+ with both coordination sites (see FIG. 5), similar to chelation with thioglycolic acid.
example 3
Sequestering Transition Metals from Aqueous Solution and Polar Organic Solvent
[0024]A series of experiments were performed to determine the ability of 1 to sequester metals (in particular Cu2+ and Pd2+) from various polar solvents, including water. Our initial studies involved sequestration of transition metal cations such as Co2+, Ni2+, Cu2+, Pd2+ and Ru3+ from solutions of their salts in deionized water, methanol or acetonitrile by a heptane solution of 1. In a typical experiment, a solution of sequestrant in heptane was added to a solution of CuSO4 in water and shaken, with resulting formation of an emulsion. Shaking was continued for 2 h. During this time, visually observed discoloration of the aqueous phase qualitatively indicated a high level of Cu2+ sequestration. Quantitative inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis of the polar phase that indicated 60-fold decrease of copper content (Table 1) confirmed this observation. A control experimen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com