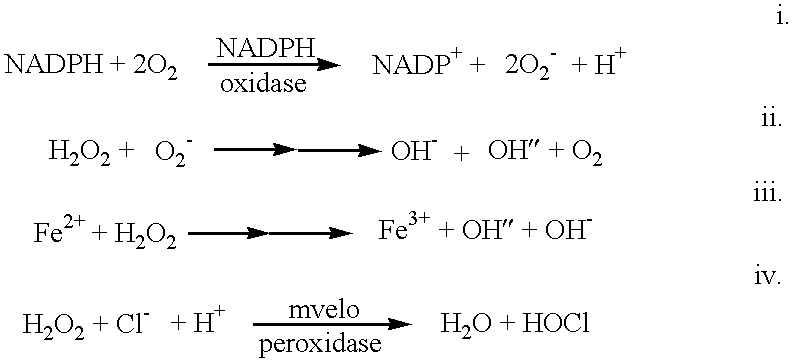
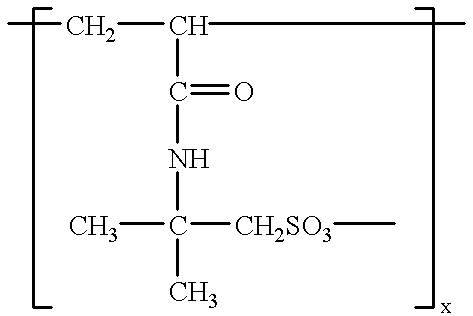
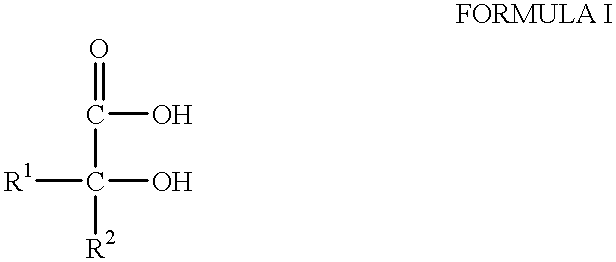Anti-inflammatory formulations for inflammatory diseases
a technology of inflammatory diseases and formulations, applied in the field of topical formulations, can solve the problems of not being particularly effective at reducing the duration of vital shedding or lesion, not being particularly effective at reducing or treating recurrent disease, and further inflammation, and achieves broad-spectrum antimicrobial activity and prevents transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
This example illustrates a formulation useful for the topical treatment of genital herpes according to the methods of the present invention. This formulation also can be used for hemorrhoids and has anti-inflammatory activity, as shown in Examples 7-9. There is prepared a two-part topical composition according to the invention, having a first gel with sodium chlorite as the chlorine dioxide liberating agent and a second gel with lactic acid as the activator protic acid. The formulations on a percent weight basis are as follows:
% First Gel Poly (sulfonic acid) 45.0 (16% solution .+-. 1%) Sodium hydroxide 1 N 45.0 Sodium chlorite (80% .+-. 5%) 0.32 Tetrasodium EDTA 0.19 Water q.s. Second Gel Lactic Acid (38% .+-. 5%) 2.64 Natrosol 150 MR 1.75 Isopropyl alcohol U.S.P. 5.0 Poloxamer 188 0.4 Sodium Benroate 0.04 Water q.s.
example 2
The composition of Example 1 was prepared for a clinical trial in a pair of unit dose sachets. A 2-gram quantity of gel containing 0.16% of active chlorite was prepared by mixing the contents of both sachets immediately prior to application. Thirty-five patients (30 males and 5 females) were enrolled. Thirty-four were diagnosed as having active genital herpes. Thirty-one patients complied with the treatment of twice daily dosing for seven days. Three patients received the compositions of Example 1 t.d.s., and one patient defaulted. Patients were examined daily until the lesions were healed (defined as re-epithelialization of the original lesions). The results of the study were compared to a similar study conducted with topical acyclovir and placebo (Fiddian et al, J. Antimicrob. Chem. 12:Suppl. B:67-77, 1983) and are presented together in Table 1 below:
Median Median Duration of Viral Median Recurrence Symptoms Shedding Healing Rate (d) Time (d) Time (d) % Example 1 3* 1** 8 (1-17) 1...
example 3
The following formulation can be used as a dermatologic gel for psoriasis treatment:
% Base Sodium chlorite (80% .+-. 5%) 0.32 Tetrasodium EDTA 0.19 Poly (sulfonic acid) 45.0 (16% solution .+-. 1%) Sodium hydroxide 1 N 40.0 Nacconol 90F 1.8 Water q.s. Activator Propylene glycol U.S.P. 40.0 Salicylic acid U.S.P. 2.0 Poloxamer 188 0.4 Sodium Benzoate 0.04 Natrosol 250 MR 2.1 Isopropyl alcohol U.S.P. 5.0 Water q.s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com