Method for preparing acephate
The technology of an acephate and a new method is applied in the field of pesticide preparation, can solve the problems of low total separation yield, reduced product yield and purity, separation difficulty, etc., and achieves overcoming neutralization process and reducing decomposition loss. , the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
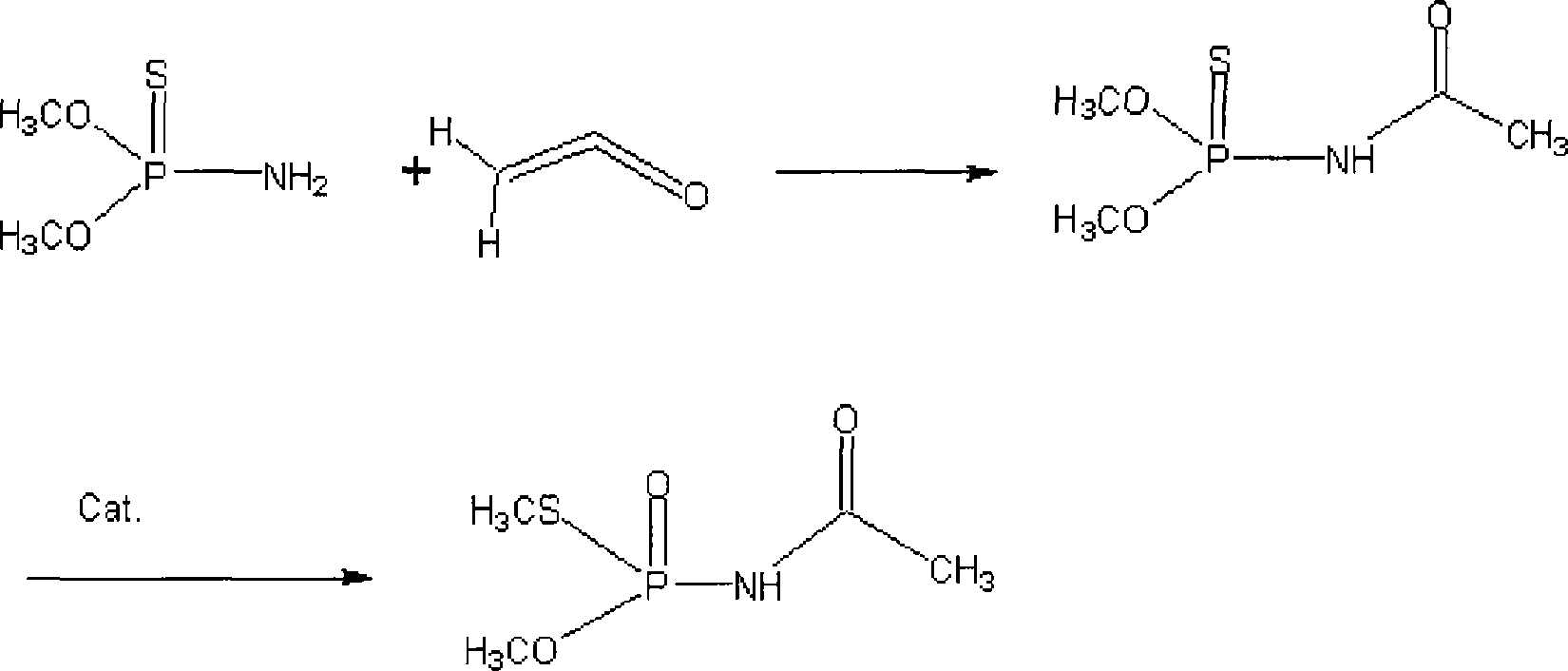
[0035] Embodiment 1: in the 1000mL four-necked flask that has stirrer, condenser, thermometer and vent tube, add the O of 303g (93%), O-dimethylphosphorothioate, 300mL dichloroethane and 1.8 g of p-toluenesulfonic acid, under vigorous stirring, controlled temperature is 20 ℃ with ice brine, feed the newly produced ketene and nitrogen gas mixture, control the speed of about 40-50g / hour to feed ketene, when the reaction mixture is no longer The reaction stops when ketene is absorbed, and the reaction time is 4-6 hours.
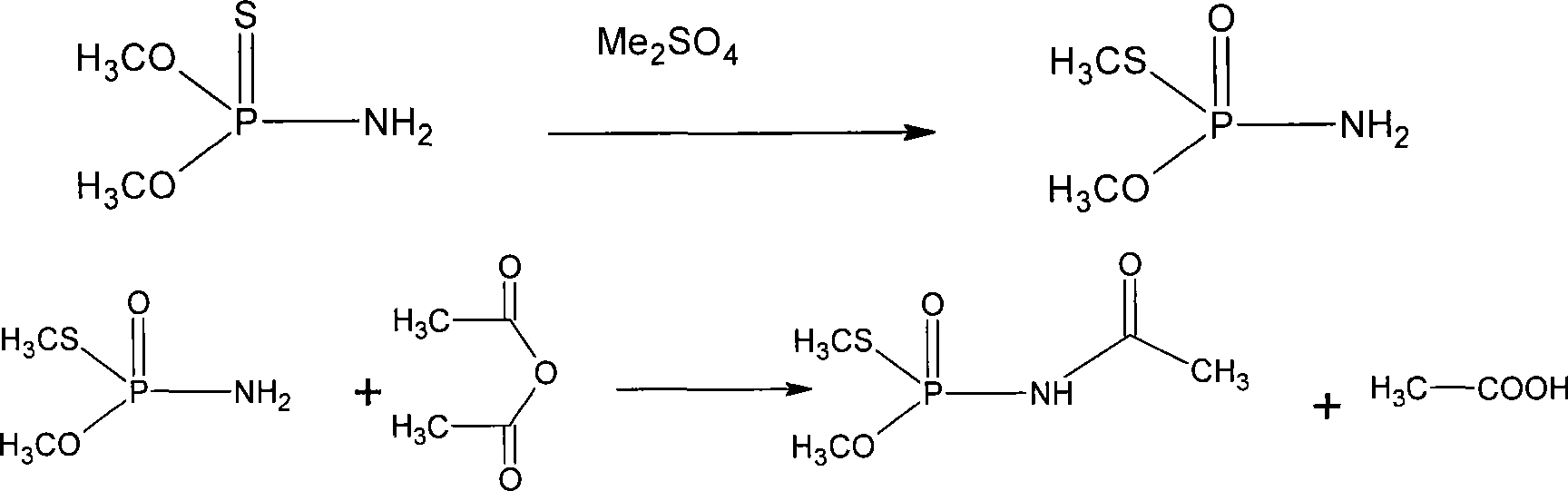
[0036] Carry out isomerization reaction: add 20 g of dimethyl sulfate and 2 g of methyl iodide to the above reaction product, and carry out isomerization reaction at 50-65° C. for 4-5 hours under stirring to obtain acephate stock solution. Be cooled to 0-5 ℃, separate out a large amount of solids, filter, mother liquor can be circulated to next batch of reaction solution, wash product with 100ml ethylene dichloride, vacuum-dry, obtain acephate 245g yield and be ...
Embodiment 2
[0037] Embodiment 2: in the 1000mL four-necked flask that has stirrer, condenser, thermometer and ventilation tube, add the O of 303g (93%), O-dimethyl phosphorothioate, 300mL dimethyl carbonate and 1.5 g boron trifluoride-diethyl ether, under vigorous stirring, control the temperature with ice brine to 20°C, feed the newly produced mixed gas of ketene and nitrogen, control the speed of about 40-50g / hour to feed ketene, when the reaction mixture Stop the reaction when ketene is no longer absorbed, and the reaction time is 4-6 hours.
[0038]Carry out isomerization reaction: add 20 g of dimethyl sulfate and 2 g of methyl iodide to the above reaction product, and carry out isomerization reaction at 50-65° C. for 5-6 hours under stirring to obtain acephate stock solution. Be cooled to 0-5 ℃, separate out a large amount of solids, filter, mother liquor can be circulated to next batch of reaction solution, wash product with 100ml ethylene dichloride, vacuum-dry, obtain acephate 232...
Embodiment 3
[0039] Embodiment 3: in the 1000mL four-necked flask that has stirrer, condenser, thermometer and ventilation tube, add the O of 303g (93%), O-dimethyl phosphorothioate, 300m ethyl acetate and 3g sulfuric acid , under vigorous stirring, control the temperature of 20°C with ice brine, feed the newly produced mixed gas of ketene and nitrogen, control the speed of about 40-50g / hour to feed ketene, and stop when the reaction mixture no longer absorbs ketene Reaction, the reaction time is 4-6 hours.
[0040] Carry out the isomerization reaction:
[0041] Add 20 g of dimethyl sulfate and 2 g of methyl iodide to the above reaction product, and carry out isomerization reaction at 50-65° C. for 4-5 hours under stirring to obtain acephate stock solution. Be cooled to 0-5 ℃, separate out a large amount of solids, filter, mother liquor can be circulated to next batch of reaction solution, wash product with 100ml ethylene dichloride, vacuum-dry, obtain acephate 235g yield and be 64.2% (wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com