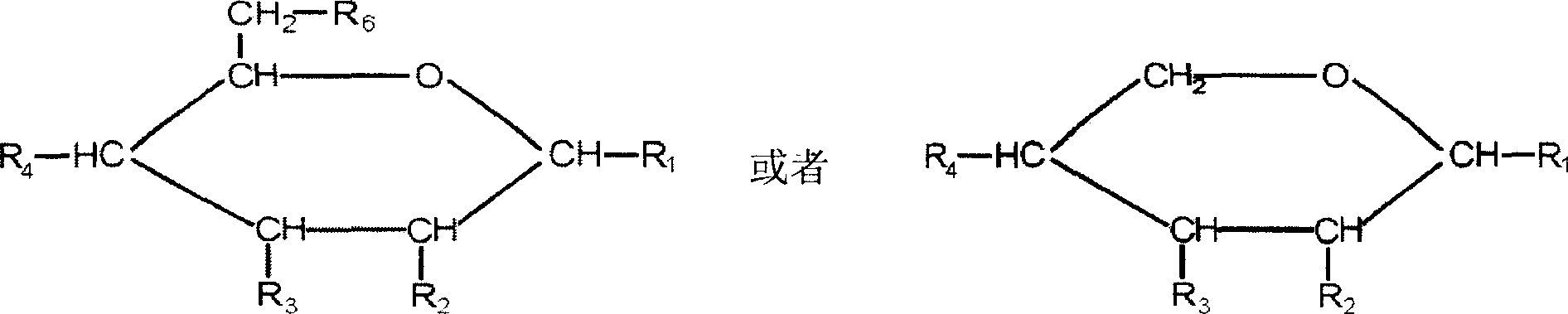
Polysaccharide sulfate selenide compound and its preparation method and application
A technology of polysaccharide sulfate and selenized polysaccharide, which is applied in the direction of drug combination, antineoplastic drugs, etc., can solve the problems of cumbersome preparation and low selenium content, and achieve the effect of a wide range of physiological functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 In vitro detection of selenated polysaccharide sulfate anticancer drug susceptibility test by MTT method
[0033] Cells in the logarithmic growth phase were diluted to 2.0×106 cells mL-1 with RPMI-1640 medium containing 10% fetal bovine serum, inoculated in a 96-well plate, 100 μL per well, and then added the drug to be tested and the negative control in sequence group, positive control group, and keep the blank group with medium only. Subsequently, culture was continued for 24 hours in an incubator at 37° C. and 5% CO 2 , and then 10 μL of 5 mg·mL −1 MTT solution was added to each well, and culture was continued for 4 hours. Aspirate the supernatant, add 100 μL of DMSO to each well, and measure the absorbance (A) value with a microplate reader at a wavelength of 570 nm. Calculate the cell proliferation inhibition rate with the following formula, and use the formula T / C (%)=100×A 阴性 / A 样品 The half inhibitory concentration (IC50) inhibitory rate=(A 空白 -A 给...
Embodiment 2
[0035] Embodiment 2 antioxidant activity research
[0036] 1. to O 2 · The scavenging effect
[0037] Contains selenated polysaccharide sulfate (1mg·mL -1 ) Tris-HCl buffer solution of pH 8.2 was incubated at 25°C for 20 minutes, and 0.1mol·L -1 10mmol·L of pyrogallol -1 Hydrochloric acid solution, shake quickly, measure the absorbance at 319nm at 25°C, and continuously measure A p .
[0038] Do not add selenized polysaccharide sulfate, operate as above, measure its contrast absorbance value A s . The test showed that the absorbance A p Relative Absorbance A s reduce.
[0039] The results showed that: selenized polysaccharide sulfate can effectively scavenge active oxygen.
[0040] 2. Scavenging effect on hydroxyl radicals and protecting DNA
[0041]Using Fenton system, take 0.2mL F eSO4-EDTA mixed solution (10mmol L -1 ) into the test tube, add 0.5mL of DNA (10mmol·L -1 ), then add selenated polysaccharide sulfate (1mg·mL -1 ), with phosphate buffer (pH 7.4, 0.1...
Embodiment 3
[0044] The chemical synthesis of embodiment 3 polysaccharide sulfate.
[0045] in N 2 Under protection, 1 g of polysaccharide was added into 150 mL of tetrahydrofuran (N, N-dimethylformamide, DMF), and stirred at room temperature for 14 h. N 2 Under protection, sulfur trioxide·pyridine was added while stirring (the molar ratio of sulfur trioxide·pyridine to hydroxyl groups in the polysaccharide was 10:1), and then reacted at 40°C for 10 h. Crude polysaccharide sulfate pyridinium salt was prepared by treating with 500 ml of methanol and stirring for 1 hour. Filter with a sintered glass funnel equipped with an aspirator, and wash with methanol continuously until the filtrate becomes colorless, and dry in the air. The crude polysaccharide sulfate pyridinium salt was dissolved in 6.5 ml of 30% sodium acetate solution; 7 ml of double distilled water was added to replace the pyridinium salt with sodium salt. Then the salt solution was slowly added into 100 ml of anhydrous methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com