Preparation method of 2-chloronicotinic acid
A technology of chloronicotinic acid and chlorinated reagents, applied in the direction of organic chemistry, can solve the problems of waste pollution, difficult production, large dosage, etc., and achieve the effects of high oxidation yield, light environmental pollution, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
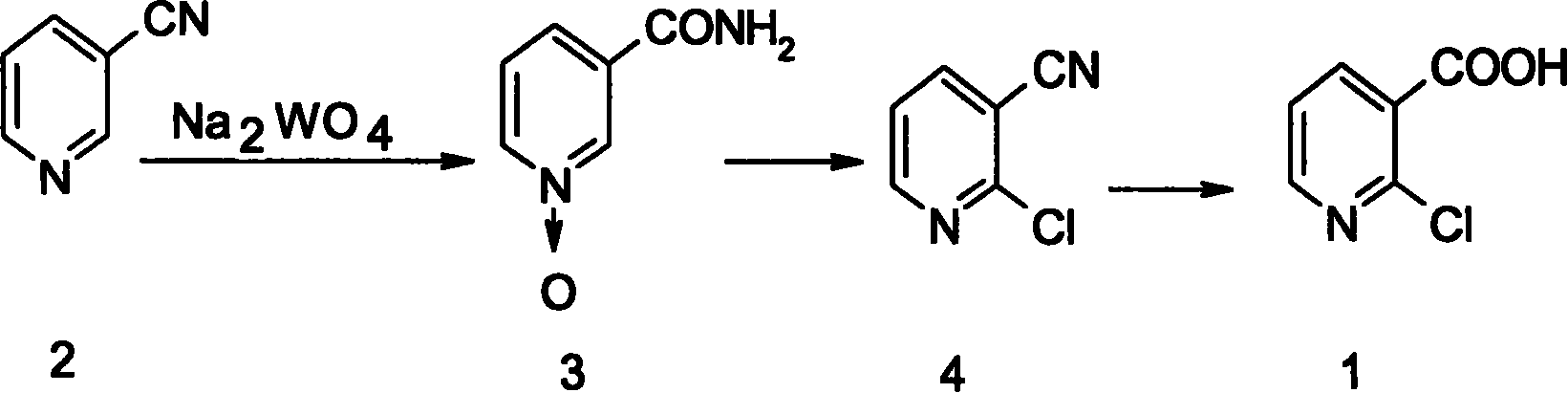
[0016] Example 1 Preparation of nicotinic acid amide N-oxide (3)
[0017] In a 1000mL three-necked round-bottomed flask, equipped with a thermometer and a constant pressure dropping funnel, put in 200g 3-cyanopyridine, 0.2g molybdenum acetylacetonate, 40ml water, stir, heat the water bath to 65℃, and add dropwise within 1 hour 30% hydrogen peroxide 50g, keep for 0.5 hour; add 30% H dropwise within 2 hours 2 O 2 110g, keep for 0.5 hour; add 30% H dropwise within 2 hours 2 O 2 110g, keep holding for 4 hours. Use starch KI test paper to test the complete decomposition of hydrogen peroxide, HPLC analysis of raw material reaction below 1%, add 20ml saturated Na dropwise 2 CO 3 Measure the pH value to 8, control the temperature below 65°C, recover 200ml of water, cool to 0°C in an ice-salt bath, let stand for half an hour and filter, wash the filter cake with 50ml deionized water, and dry it to get 252g, with 95% Yield Preparation of niacinamide N-oxide (3).
Embodiment 2
[0018] Example 2 Preparation of nicotinic acid amide N-oxide (3)
[0019] In a 500mL three-necked round-bottomed flask, equipped with a thermometer and a constant pressure dropping funnel, put 104g 3-cyanopyridine, 0.1g molybdenum acetylacetonate, 20ml water, stir, and heat up the water bath to 50℃, at a constant speed within 6 hours 340 g of 20% hydrogen peroxide was added dropwise, and the temperature was kept for 4 hours. Use starch KI test paper to test the complete decomposition of hydrogen peroxide, HPLC analysis of the reaction of raw materials below 1%, drip with 20ml saturated K 2 CO 3 Measure the PH value of 8.5, control the temperature at about 60℃, recover 150ml of water, cool to -5℃ in an ice-salt bath, let stand for half an hour and filter, wash the filter cake with 30ml of deionized water, and dry it to obtain 128.3g. % Yield to prepare niacinamide N-oxide (3).
Embodiment 3
[0020] Example 3 Preparation of 2-chloro-3-cyanopyridine (4)
[0021] Nitrogen positive pressure protection, dry and anhydrous 1000mL four-neck round bottom flask are equipped with a thermometer, two constant pressure dropping funnels, one of the constant pressure dropping funnel and the reflux condenser have the same Y interface, and the upper end of the condenser is connected Calcium chloride drying tube, escaping acid mist and absorbing with sodium hydroxide aqueous solution. Add 138g nicotinic acid amide N-oxide (3), 2.7g phenylphosphoryl dichloride, 140ml chloroform, freezing in an ice salt bath -5℃, 63g pyridine, 170g POCl 3 Put them in a constant pressure funnel, control the temperature at -5~5℃, and add pyridine and POCl dropwise at the same time 3 , After about 2 hours of dripping, heat up to 35°C, hold for 1 hour, heat up to 55°C, hold for 1 hour, heat up to 65°C, keep refluxing for 4 hours, recover chloroform under normal pressure, and recover excess POCl under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com