Method for separating dimethyl carbonate and methanol azeotrope
A technology of dimethyl carbonate and azeotrope, which is applied in the field of steam permeation membrane separation technology to separate dimethyl carbonate and methanol azeotrope, which can solve the problem of reducing membrane area, large load of methanol recovery tower, large heat load of extraction water, etc. Problems, to achieve the effect of small concentration polarization, improve safety and reliability, and increase operating temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
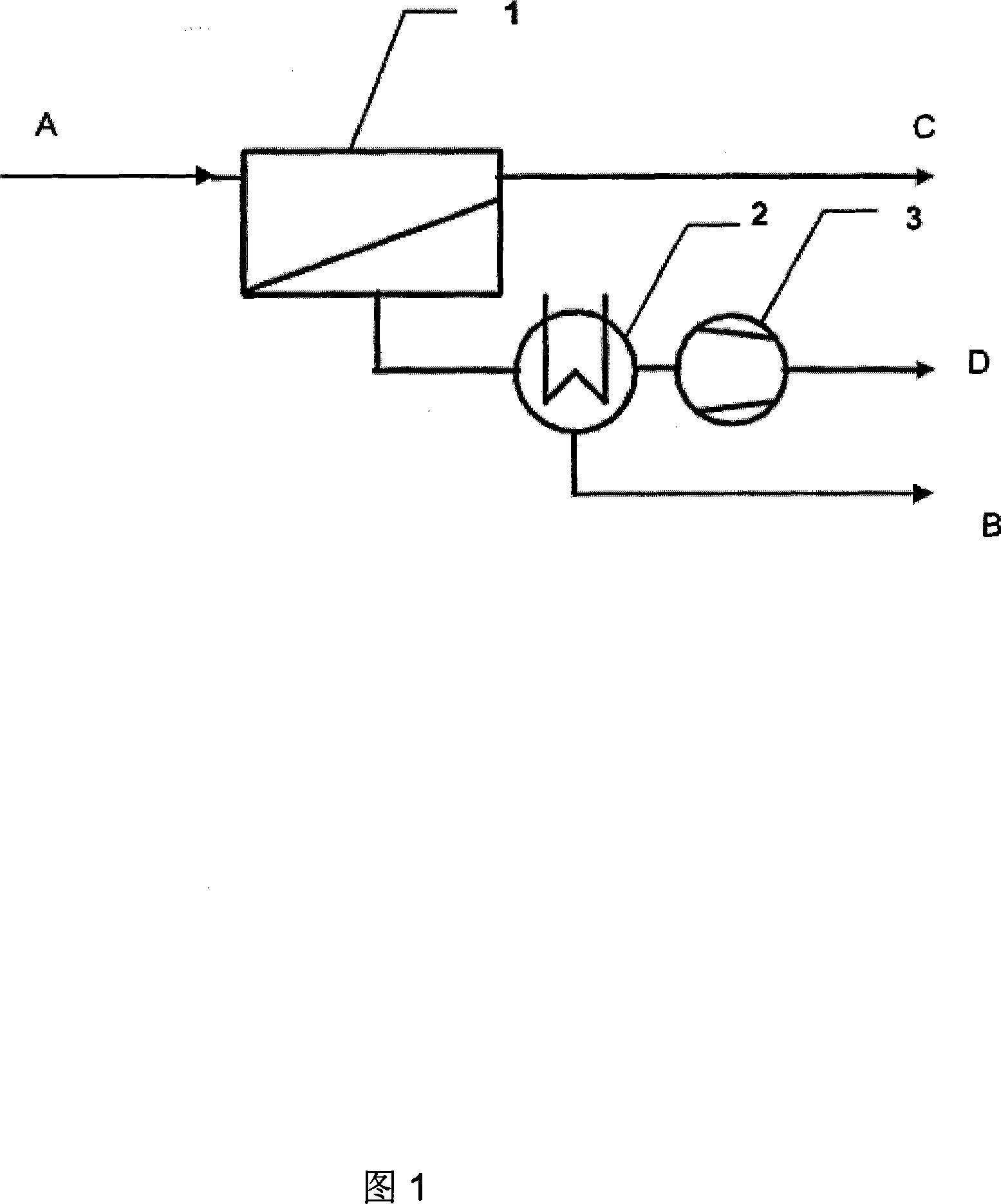
[0023] Take dimethyl carbonate and methanol azeotrope as vapor feed A, dimethyl carbonate content 30% (wt), methanol content 70% (wt), mass flow rate is 25kg / h, feed pressure is 0.4Mpa, enters The temperature of the material is 105°C, and it enters the membrane separator 1 to contact with the GKSS POMS-silicalite / PEI composite membrane. The downstream side of the membrane separator is evacuated with a vacuum pump 3 to maintain the vacuum at 75mmHg absolute pressure. Ester molecules have selective passability, and dimethyl carbonate molecules pass through the membrane preferentially driven by the vapor partial pressure difference on both sides of the membrane, and are enriched on the permeation side of the membrane, and condensed into liquid in the condenser 2 under vacuum and low pressure. The dimethyl carbonate content in the mixture B is 56% (wt) through gas chromatography analysis, and the dimethyl carbonate product can be further purified by conventional rectification to ob...
Embodiment 2
[0025] Take dimethyl carbonate and methanol azeotrope as vapor feed A, dimethyl carbonate content 30% (wt), methanol content 70% (wt), mass flow rate is 25kg / h, feed pressure is 0.1Mpa, enters The material temperature is 64°C, and it enters the membrane separator 1 to contact with the GKSS POMS / PEI composite membrane. The downstream side of the membrane separator is evacuated with a vacuum pump 3 to maintain a vacuum degree of 25mmHg absolute pressure, and the separation membrane is effective against the dimethyl carbonate molecules in the raw material. With selective passability, dimethyl carbonate molecules pass through the membrane preferentially driven by the difference in vapor partial pressure on both sides of the membrane, and are enriched on the permeate side of the membrane, and are condensed into liquid in the condenser 2 under vacuum and low pressure. The liquid mixture B The middle dimethyl carbonate content is 40.5% (wt) through gas chromatographic analysis, across...
Embodiment 3
[0027] Take dimethyl carbonate and methanol azeotrope as steam feed A, dimethyl carbonate content 25% (wt), methanol content 75% (wt), mass flow is 25kg / h, feed pressure is 0.4Mpa, enters The temperature of the material is 105°C, it enters the membrane separator 1 and contacts with GKSSPOMS / PEI, and the downstream side of the membrane separator is evacuated with a vacuum pump 3 to maintain the vacuum at 10mmHg absolute pressure, and the separation membrane selectively passes through the dimethyl carbonate molecules in the raw material Dimethyl carbonate molecules preferentially pass through the membrane driven by the vapor partial pressure difference on both sides of the membrane, enrich on the permeate side of the membrane, and condense into liquid in condenser 2 under vacuum and low pressure. Dimethyl carbonate in the liquid mixture B Methyl ester content is 40% (wt) through gas chromatographic analysis, across the azeotropic composition, can be further purified by convention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com