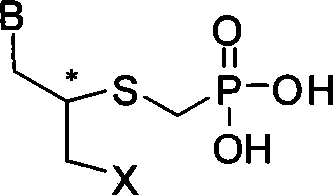
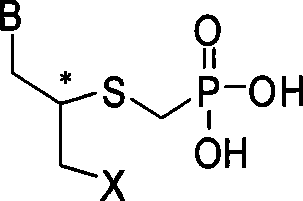
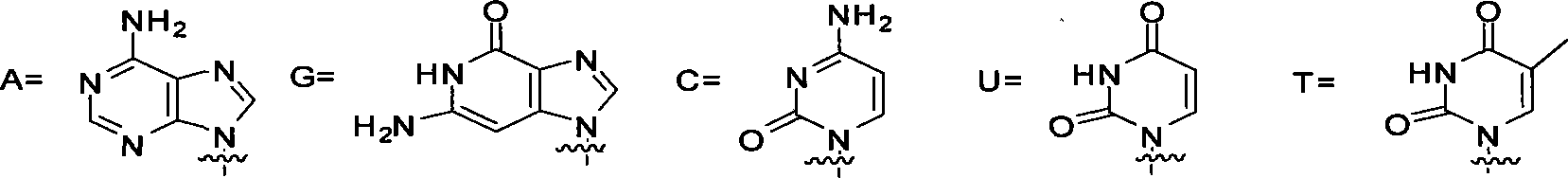
Sulfur-containing ring-free nucleosides phosphonate analogue and preparation method thereof
A technology of nucleoside phosphonic acid and analogues, which is applied in the field of medicine and chemical industry, can solve problems not in the research field of nucleoside phosphonic acid analogues, and achieve the effects of easy-to-obtain raw materials, high yield, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 (S)-9-N-(2-phosphonomethylene mercaptopropyl)-adenine synthesis
[0030] 1. Synthesis of (R)-propylene carbonate
[0031] Add (R)-1,2-propanediol (1eq), diethyl carbonate (1.5eq), sodium methoxide (0.02eq) into the flask, heat up and distill under normal pressure, when the ethanol evaporated is about 1eq, distill under reduced pressure , the 95-96°C / 3mmHg fraction was collected to give (R)-propylene carbonate (76.2%) as a colorless liquid. 1 H NMR (CDCl 3 ): δ4.811 (m, 1H, OCH 2 CHCH 3 ), δ4.518(t, 1H, OCH 2 CHCH 3 ), δ3.983(t, 1H, OCH 2 CHCH 3 ), δ1.431 (d, 3H, OCH 2 CHCH 3 ).
[0032] 2. Synthesis of diisopropyl p-toluenesulfonyloxymethylphosphonate
[0033] Diisopropyl phosphite (1eq), paraformaldehyde (1.2eq), and TEA (0.5ml) were refluxed for 3h, then cooled to 0°C, p-toluenesulfonyl chloride (0.9eq) and TEA (6ml) were added slowly, After adding, it was raised to room temperature. After reacting for 4h, diethyl ether (20ml) was added, filt...
Embodiment 2
[0040] Example two (R)-9-N-(2-phosphonomethylene mercaptopropyl)-adenine synthesis
[0041] (R)-9-N-(2-phosphonomethylenemercaptopropyl)-adenine can be obtained (56%) by using (R)-1,2-propanediol as raw material and referring to the conditions of Example 1.
Embodiment 3
[0042] Example 3 Synthesis of (R)-1-N-[(2-phosphonomethylene mercapto-3-hydroxyl-propyl)]-cytosine
[0043] 1. Synthesis of (R)-benzylglycidol
[0044] Under the protection of N2, add (R)-glycidol (1eq) dropwise to the NaH (1eq) / DMF suspension in an ice bath, stir for 30min, then add BnCl (1.2eq) dropwise, naturally rise to room temperature, and stir for 2.5h Stop the reaction, remove the DMF solvent, and use ethyl acetate / water solution. The organic layer was washed with saturated NaHCO 3 solution, saturated NaCl solution and washed with anhydrous NaCl 2 SO 4 After drying, a pale yellow liquid (55%) was obtained.
[0045] 2. Synthesis of (R)-1-N-[(2-hydroxyl-3-benzyloxy)propyl]cytosine
[0046] N 2 Under protection, add cytosine (1eq), anhydrous DMF, NaH (0.6eq) into the flask, stir for 1.5h, add (R)-benzylglycidol (0.9eq), react at 110°C for 3h, remove DMF, Extract with dichloromethane / tert-butanol / water, and extract the aqueous layer with dichloromethane / tert-butanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com