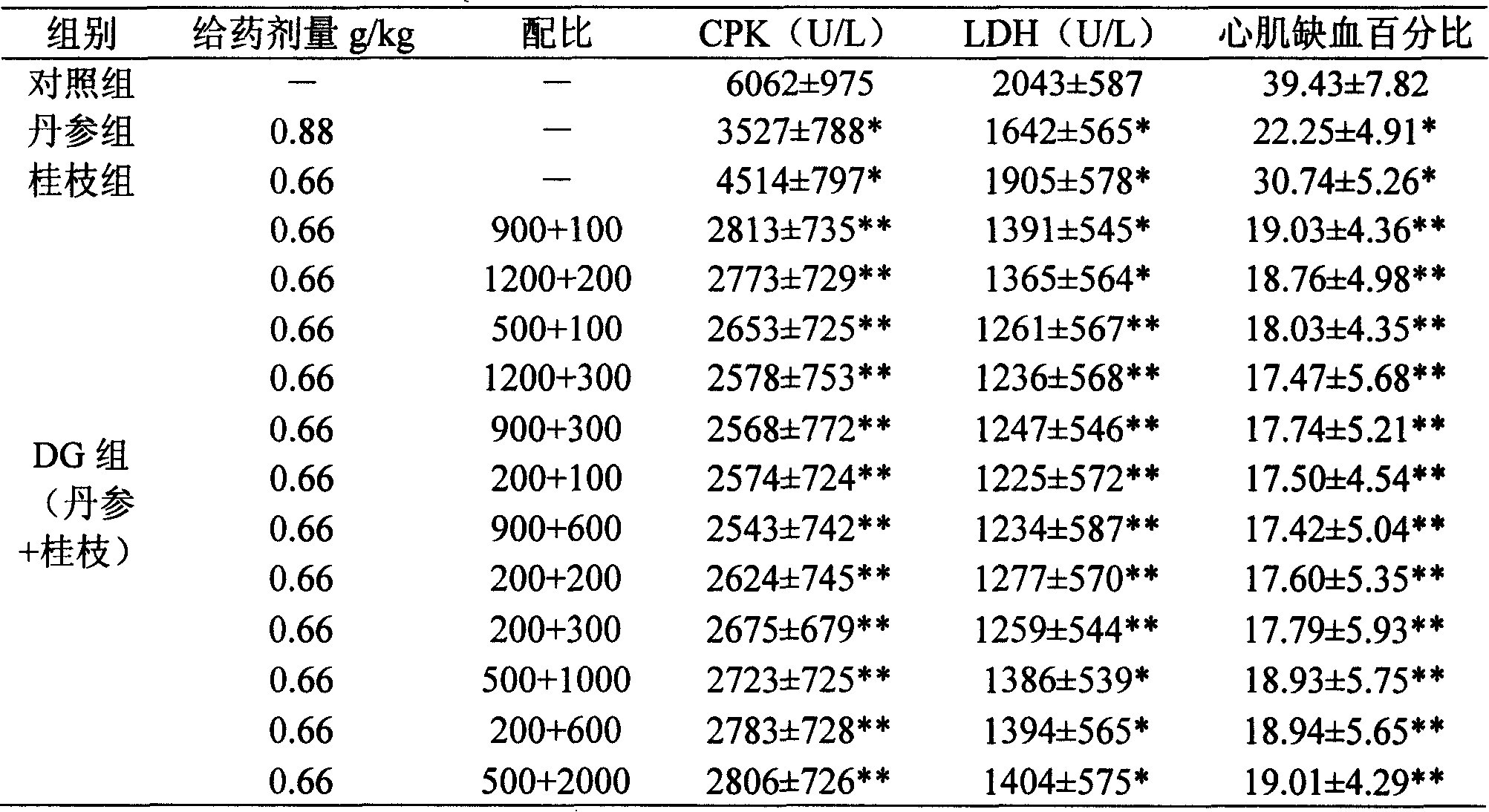
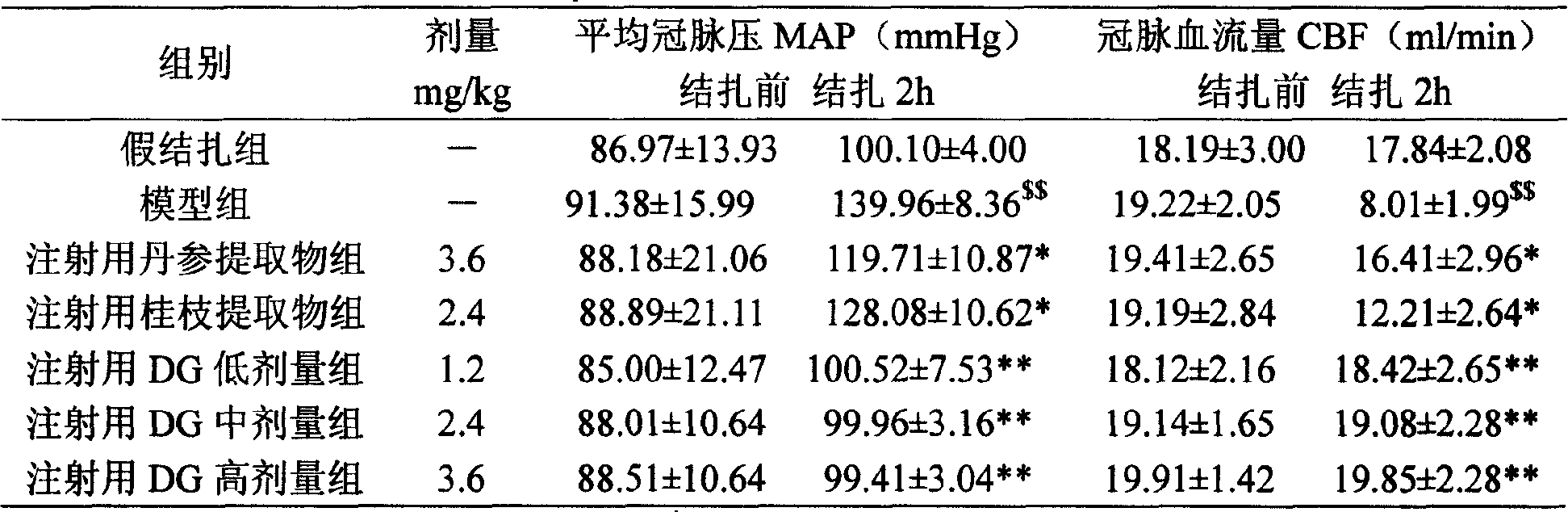
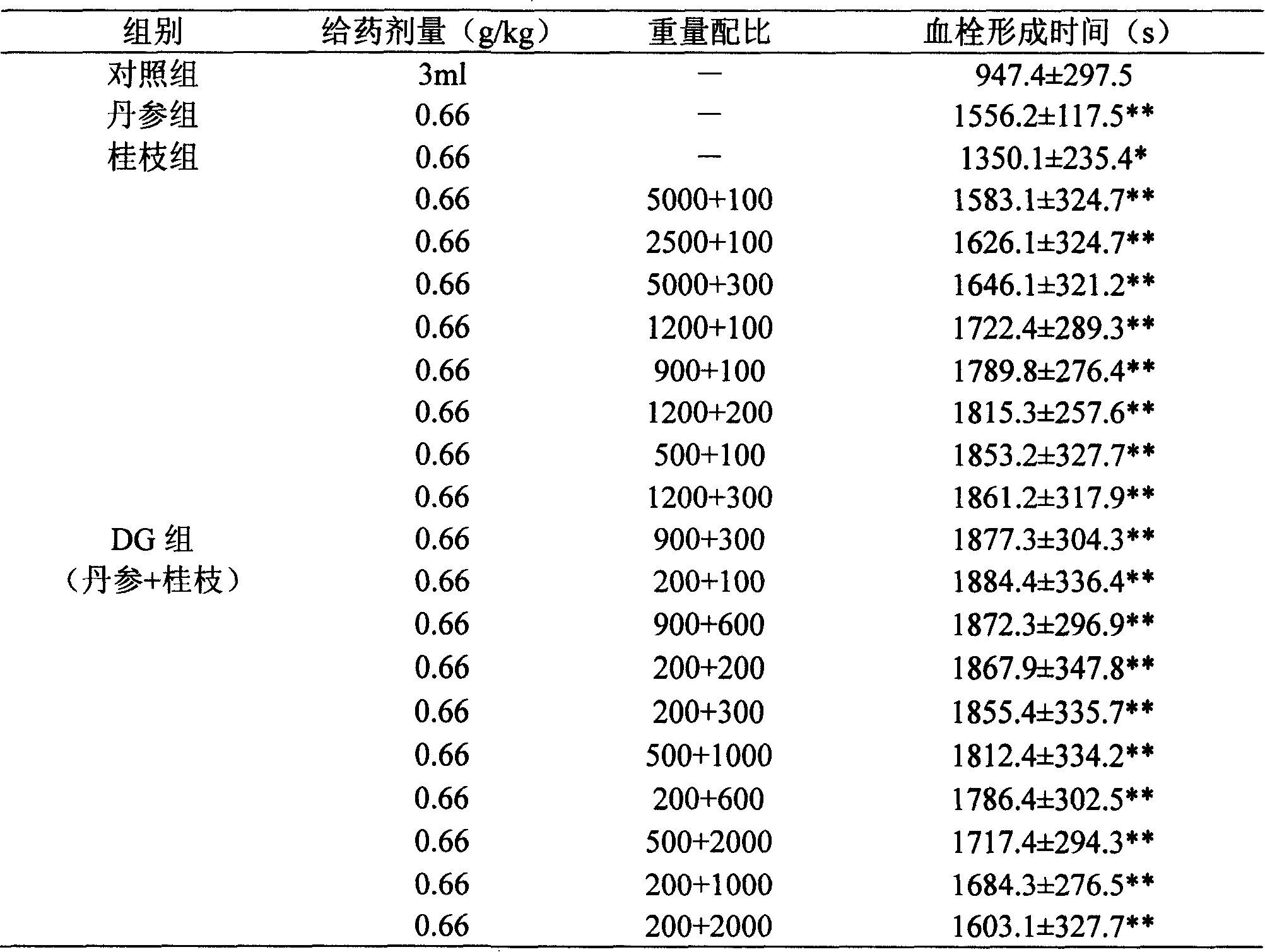
Pharmaceutical composition of red sage root and cassia twig
A composition and drug technology, applied in the field of medicine, to achieve the effects of prolonging the time of thrombus formation, good stability of preparations, and controlling product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Preparation and assay of embodiment 1 salvia miltiorrhiza extract
[0088] 1. Preparation of Salvia Miltiorrhiza Extract
[0089] Take Danshen medicinal material, crush it into coarse powder, add water to decoct twice, add 12 times of water for the first time, add 10 times of water for the first time, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate to relative density under reduced pressure 1.10~1.15 (60°C) concentrated solution, add ethanol until the alcohol content reaches 85%, refrigerate for 24 hours, filter, recover the ethanol from the filtrate until it has no alcohol smell, add hydrochloric acid to adjust the pH value to 2, shake with ethyl acetate Extracted 3 times, combined ethyl acetate solution, and evaporated to dryness. The residue was dissolved in acidic water with a pH value of 2, added to the treated polyamide column, first eluted with 2 times the column volume of water, discarded the water washing solution, and then...
Embodiment 2
[0103] Preparation and assay of embodiment 2 cassia twig extract
[0104] 1. Preparation of Cinnamon Twig Extract
[0105] Cinnamomum twig herbs are crushed to a certain particle size, put into the extraction kettle, and the SFE system is heated. The primary separation temperature is 55°C, the pressure is 30Mpa, the extraction temperature is 60°C, the secondary separation pressure is 20Mpa, and the temperature is 50°C. The time is 3 hours, and the material is discharged from the primary and secondary separation kettles.
[0106] 2. Determination of the content of cassia twig extract
[0107] Determination of cinnamic aldehyde content
[0108] Gas Chromatography:
[0109] Chromatographic conditions and system suitability test: polyethylene glycol (PEG)-20M was used as the stationary phase, the coating concentration was 10%; the column temperature was 190°C. The theoretical plate is not less than 3000.
[0110] Preparation of the reference substance solution: Take an appr...
Embodiment 3D
[0116] The preparation of embodiment 3DG tablet
[0117] Prescription one:
[0118] Salvia miltiorrhiza extract 18g (equivalent to 1kg of raw medicinal material)
[0119] Cinnamomum twig extract 24g (equivalent to 2kg of raw medicinal materials)
[0120] 120g pregelatinized starch
[0121] Microcrystalline Cellulose 40g
[0122] Low-substituted hydroxypropyl cellulose 40g
[0123] 2% HPMC aqueous solution appropriate amount
[0125] Carboxymethyl Starch Sodium 12g
[0126]
[0127] A total of 1000 tablets were prepared
[0128] Prescription two:
[0129] Salvia miltiorrhiza extract 32.4g (equivalent to 1800g of raw medicinal materials)
[0130] Guizhi Extract 4.8g (equivalent to 400g of raw medicinal materials)
[0131] 120g pregelatinized starch
[0132] Microcrystalline Cellulose 40g
[0133] Low-substituted hydroxypropyl cellulose 40g
[0134] 2% HPMC aqueous solution ap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com