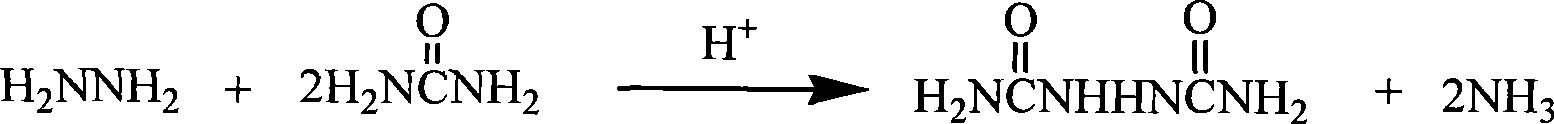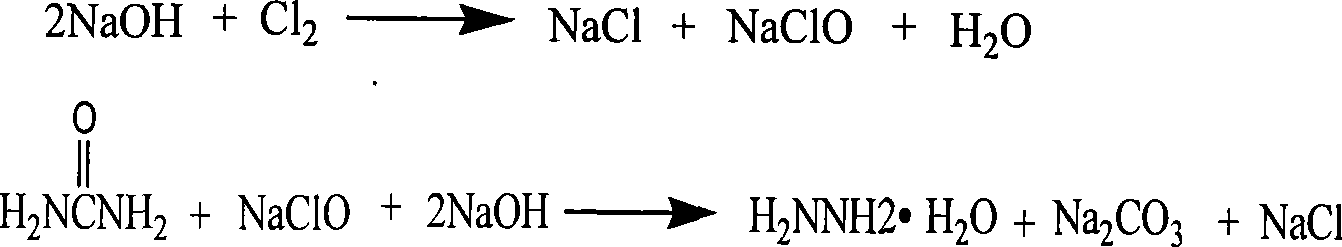Process for producing biurea
A technology of biuret and hydrazine hydrate, which is applied in the field of preparation of basic carbonate, can solve the problems of by-product pollution and high energy consumption of salt and alkali, and achieve the effects of reasonable recycling, high evaporation efficiency and increasing added value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of this biurea of the present invention, the steps are as follows:
[0025] A) Evaporate the crude hydrazine hydrate solution prepared by the urea oxidation method through a multi-effect evaporator, and after the saline-alkali content reaches 40-60%, put the above-mentioned solution into a coulter type evaporator to continue evaporating, and control the evaporation temperature at 110- 140°C until the water and hydrazine evaporate completely to obtain a salt-free hydrazine hydrate solution and a solid saline-alkali;
[0026] B) redissolve the salt and alkali released in the coulter type evaporator, react with magnesium chloride solution, zinc sulfate or chloride solution to generate basic magnesium carbonate or basic zinc carbonate precipitation, filter and wash to obtain basic magnesium carbonate or Basic zinc carbonate;
[0027] C) After dissolving the urea in the steamed hydrazine hydrate, add sulfuric acid dropwise or connect anhydrous hydro...
Embodiment 1
[0037] Using sodium hypochlorite solution with 11.5% available chlorine content and 35% urea solution according to the molar ratio of 1:1.1, react at 100-105 ℃ in the pipeline reactor, add 32% in sections in the pipeline reactor during the reaction The sodium hydroxide solution maintains sufficient alkalinity of the reaction solution, and after the reaction is completed, the content of hydrazine hydrate in the measured reactant is 5.8%.
[0038] Put the obtained crude hydrazine hydrate solution into the two-effect tubular falling film evaporator while it is hot for distillation, control the heating temperature and feed flow rate, so that the saline and alkali content of the evaporated solution is 48-50%, and the evaporated solution enters the Coulter evaporator. The hydrazine hydrate solution continues to evaporate under stirring conditions in the coulter type evaporator, and the evaporation temperature is controlled at 110-140°C until the hydrazine hydrate evaporates complete...
Embodiment 2
[0040] Take by weighing 500 grams of saline-alkali obtained in Example 1 and be mixed with 35% solution, magnesium chloride hexahydrate (Qinghai Salt Lake Industrial Group Co., Ltd.) is mixed with 30% magnesium chloride solution, under stirring condition, magnesium chloride solution is added dropwise to saline-alkali In the solution, maintain the reaction temperature at 45°C, add magnesium chloride solution dropwise according to the equivalent of sodium carbonate and sodium hydroxide in the saline solution, continue to stir for half an hour after the dropwise addition, filter with suction, wash, and dry to obtain white powdery alkali formula magnesium carbonate 265 grams. The mother liquor after suction filtration was inspected, and there was no calcium, magnesium ion and ammonia nitrogen in the solution, which met the requirements of salt electrolysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com