Radioactive sustained-release particle and preparation and use thereof
A technology of radioactivity and radioactivity, applied in the field of radiology medicine, achieves huge application advantages and prospects, increases local drug concentration, and prolongs the effect of action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: 32 Preparation of P-CP-PLLA sustained-release particles and investigation of their general shape and particle size.
[0025]Put 0.75~1.25g PLLA and 10~20mg magnesium stearate into the grinder, add 4.5~7.5ml 32 P-CP colloidal solution (radioactive concentration is 1875 ~ 5625MBq / ml), with 2ml of absolute ethanol as a dispersant, stirred at a low speed (150r / min) at a constant temperature (50°C) for 30min, placed in a vacuum oven to dry for 5h, Grind it to a powder in a grinder and press to make radioactive 32 P-CP-PLLA sustained-release particles. income 32 The specifications of P-CP-PLLA particles are as follows: the particles are light green cylindrical, with a diameter of 0.85mm-0.9mm, a length of 2.2mm-2.5mm, a round shape, tiny pores on the surface, a hard texture, and a mass of 0.9-1.1mg / granule. The radioactivity is 11.25~37.5MBq / capsule, and the single particle is packaged and sealed for storage.
Embodiment 2
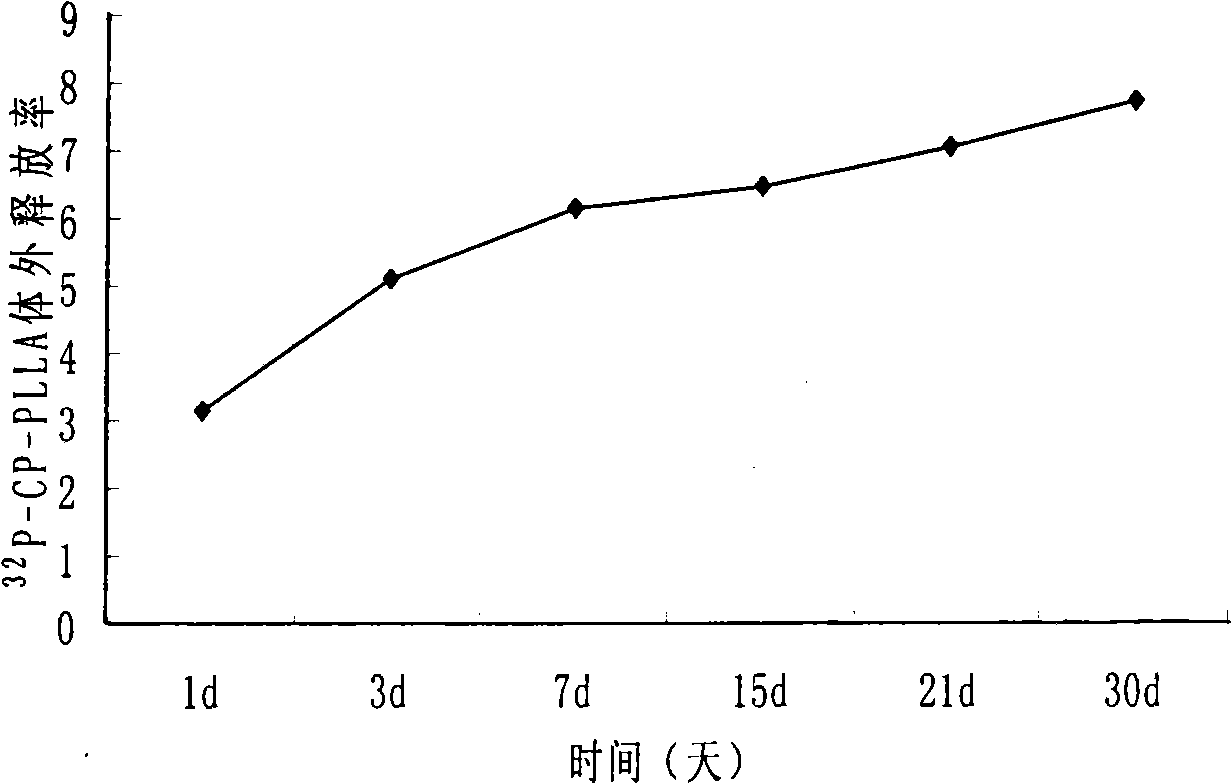
[0026] Example 2: Radioactivity 32 Study on the radioactive release rate of P-CP-PLLA sustained-release particles in vitro.
[0027] Select 90 grains with uniform mass and radioactivity 32 P-CP-PLLA slow-release particles (mass 1 ± 0.05mg, radioactivity 33.75 ± 1.6875MBq), were randomly divided into 9 groups, 10 particles in each group, and the total radioactivity of each group of particles was measured, recorded as A (n·0) (n is the group, recorded as 1-9 groups), placed in 9 petri dishes, each 3 petri dishes as a group, add release medium saline, 1640 culture solution, human serum 5.0ml each. Put it in a water bath shaker, the temperature is 37°C, and the shaking frequency is 100 times / min. On days 1, 3, 7, 15, 21, and 30 (represented by t, the time point is recorded as 1-6), 1.0ml of the release medium was taken from the culture dish, and the radioactive count was measured by an enhanced activity meter, which was recorded as B (n·t) , the radioactivity after attenuation ...
Embodiment 3
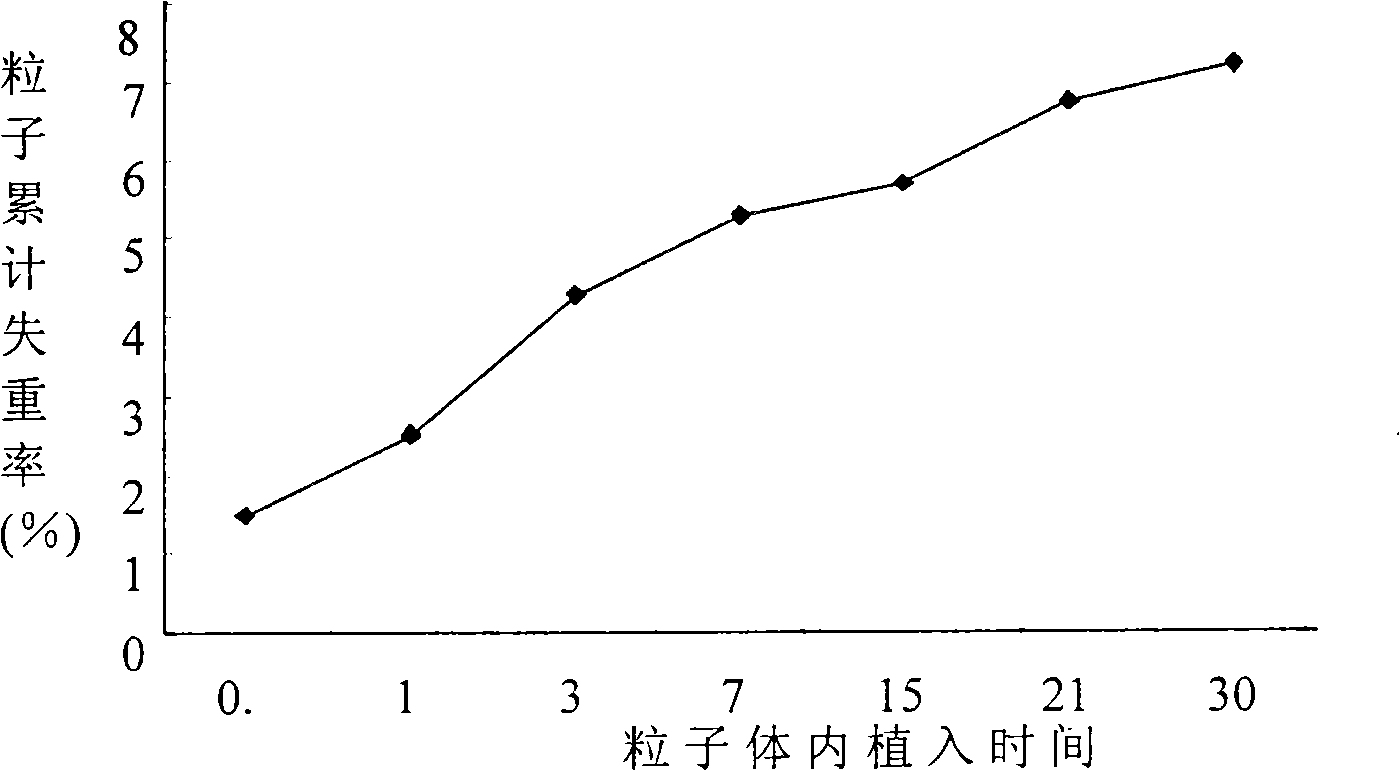
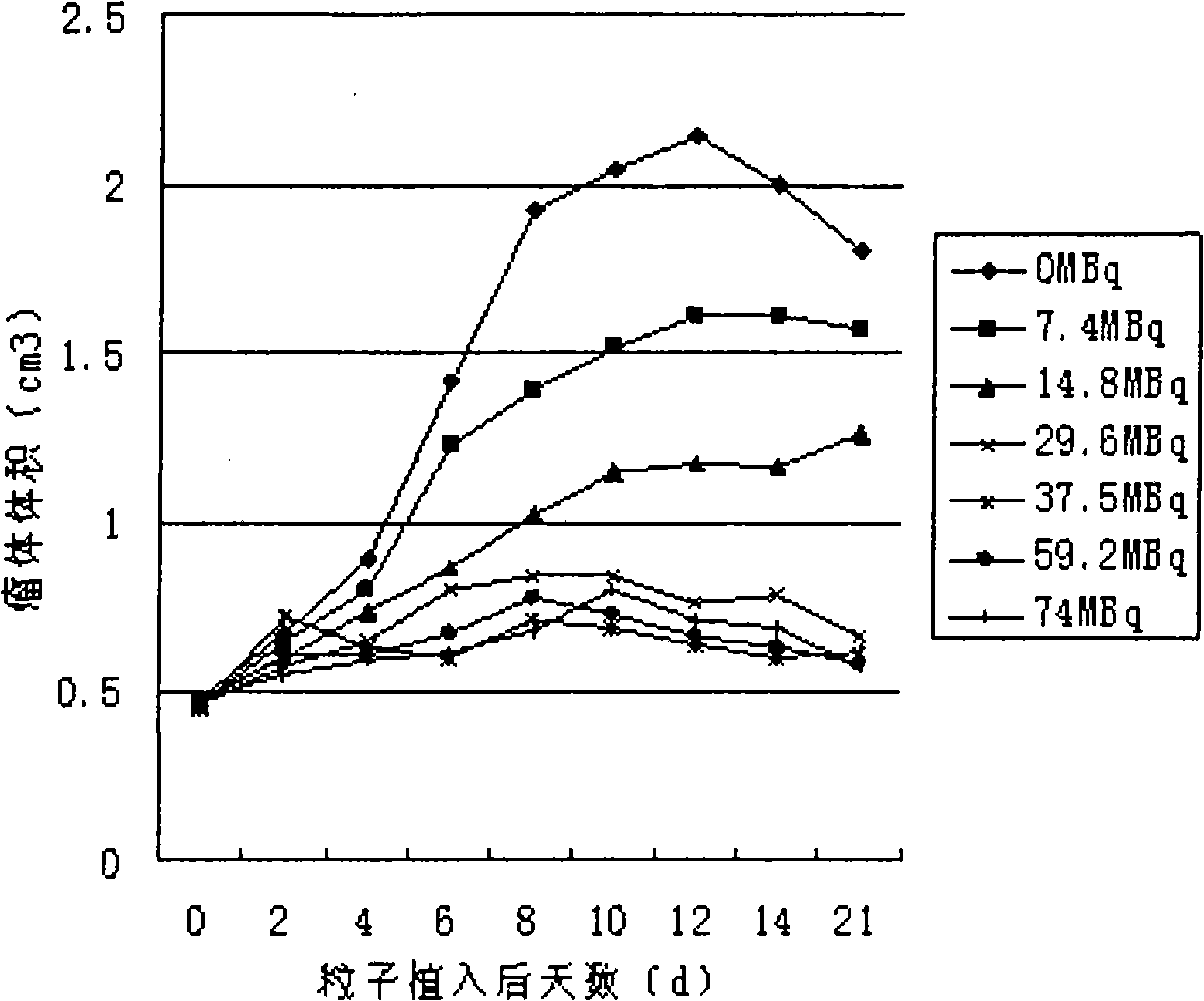
[0031] Example 3: 32 P-CP-PLLA slow-release particles implanted in mice biodegradation and 32 P Biodistribution.
[0032] Select 35 BALB / c mice, weighing 20±2g, randomly divide them into 7 groups, 5 mice in each group, measure and select the ones with more uniform mass and radioactivity 32 35 P-CP-PLLA sustained-release particles (mass 1 ± 0.05mg, radioactivity 33.75 ± 1.6875MBq), radioactive counts were measured and implanted into the left hind leg muscle of mice by percutaneous puncture, one for each mouse Dynamically observe the local and general conditions of the mice implanted with particles, and take 1ml of blood from the orbital vein of a group of mice at 0.5, 1, 3, 7, 14, 21, and 30 days after implantation, and then remove The cervical spine was sacrificed, and major organs and tissues such as the heart, liver, spleen, lung, kidney, pancreas, stomach, intestine, brain, right hind leg muscle, muscle around the particle, and femur were harvested, the wet weight was mea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Quality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com