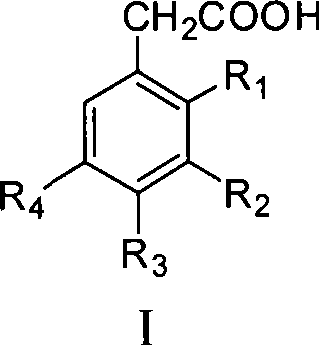
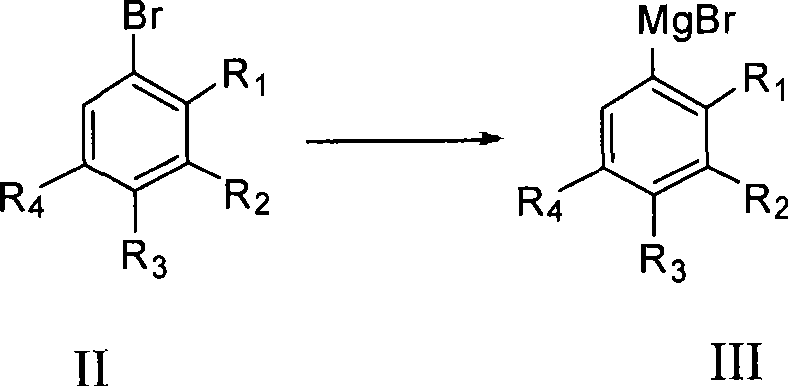
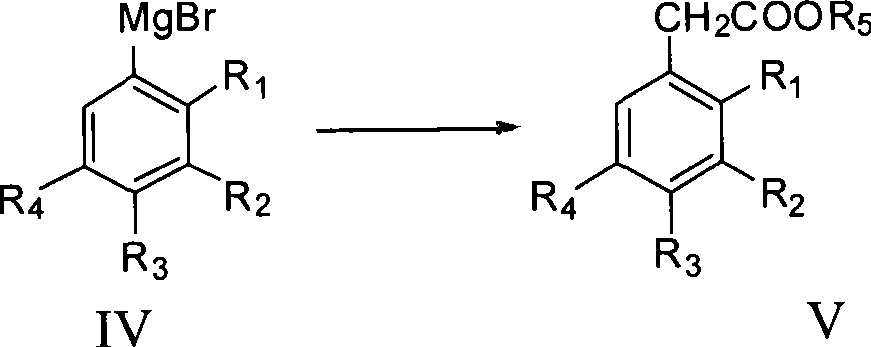
Process for producing trifluoro benzene acetic acid
A technology of trifluorophenylacetic acid and trifluorophenylacetic acid ester, which is applied in the field of preparation of trifluorophenylacetic acid, can solve the problems of reduced total yield, difficult product quality control, multiple pollutants, etc., and achieves mild reaction conditions and high product quality. The effect of stable quality and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of trifluorophenylacetic acid of the present invention: take trifluorobromobenzene as raw material and carry out Grignard reaction with Mg to obtain Grignard reagent, and then react Grignard reagent with bromoacetate in the presence of catalyst to obtain trifluorophenylethyl Ester, and then hydrolyzed under acidic or alkaline conditions to obtain the crude product of trifluorophenylacetic acid, and the content of trifluorophenylacetic acid obtained through post-treatment is ≥99%.
[0031] Beneficial effects of the present invention: the preparation method of trifluorophenylacetic acid of the present invention has easy-to-obtain raw materials, low cost, mild reaction conditions, simple and convenient operation, environment-friendly, and easy industrialization. The product quality is stable and suitable for large-scale industrial production.
Embodiment 1
[0034] Example 1 Preparation of ethyl 3,4,5-trifluorophenylacetate.
[0035] Add THF 200ml, Mg 26.4g (1.2mol) into a 500ml four-necked bottle, and add dropwise a solution of 3,4,5-trifluorobromobenzene 211g (1mol) and 150ml THF at about 30°C while stirring. Insulate and stir for 0.5 to 1 hour to end the reaction, the Grignard reagent is prepared, and insulate for later use. Put THF 250ml, CoCl 2 6.5g (0.05mol), 5.8g (0.05mol) of tetramethylethylenediamine. Stir at around 30°C for 15 minutes, cool down to -10-0°C, and slowly add Grignard reagent dropwise. After the dropwise addition, keep it warm for 0.5h, add 10% HCl dropwise below 10°C, adjust the pH to 3, remove the solvent, evaporate THF, and wash the residue with water until neutral to obtain 3,4,5-trifluorobenzene Ethyl acetate crude product 335.3g, content 57.76%, yield 88.8%. The boiling point is 103°C / mmHg.
Embodiment 2
[0036] Example 2 Preparation of 3,4,5-trifluorophenylacetic acid.
[0037] Put 3,4,5-trifluorophenylacetate ethyl 335.3 (content 57.76%, 0.888mol) and 20% NaOH aqueous solution 500g (2.5mol) into a 500ml four-necked bottle, heat up at 60-70°C for 2-3 hours, cool down Add 50ml of toluene to below 50°C and stir for 15 minutes, then let it stand for stratification, wash the water phase with 50ml of toluene*2 and put it back into the reaction bottle, add 30% hydrochloric acid dropwise below 30°C to adjust the pH of the system to 1, cool down to about 0°C and keep it warm for 30 minutes , suction filtration, the filter cake is 3,4,5-trifluorophenylacetic acid, washed with ice water, and dried to obtain 136g of the product, with a content of 99.5%, a yield of 80.6%, and a total yield of 71.6%. The boiling point is 131°C / 25mmHg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com