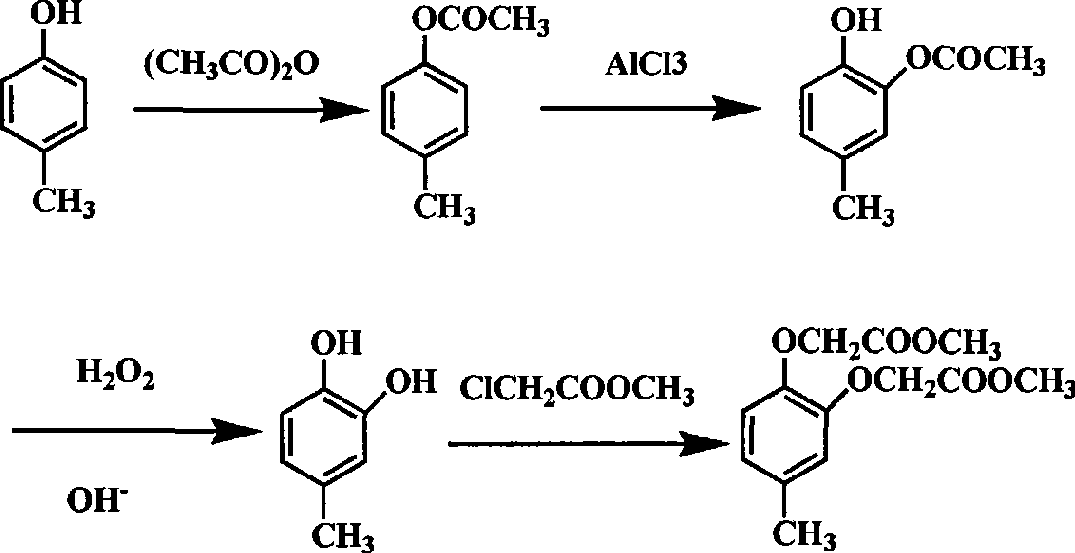
Chemical synthetic method for para-methyl catechol diacetoxyl dimethyl ester
A technology for the synthesis of methyl catechol diacetate dimethyl ester and its synthesis method, which is applied in the fields of chemical instruments and methods, organic chemistry, carboxylate preparation, etc., can solve the problems of difficult control of reaction conditions and easy side reactions, etc., and achieve The effect of low equipment requirements, low production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) After mixing p-cresol, acetic anhydride and concentrated sulfuric acid at a molar ratio of 1:1:0.001, reflux reaction under stirring for 0.5h, after the reaction, distill off the generated acetic acid and excess acetic anhydride at 150°C to obtain phenol ester;
[0017] 2) At 60°C, add anhydrous aluminum trichloride to the phenolic ester, continue stirring for 0.5h, add 4mol / L hydrochloric acid at 50°C for hydrolysis reaction, and separate the upper layer of organic layer, the lower aqueous layer was extracted with benzene, all organic layers were kept and combined, and concentrated under reduced pressure to obtain phenolic ketone 2-hydroxyl-5-methylacetophenone, wherein the molar ratio of phenolic ester and aluminum chloride was 1:1 , The molar ratio of phenol ester to hydrochloric acid is 1:1~10.
[0018] 3) Dissolving 2-hydroxy-5-methylacetophenone in 3 times the mass of ethanol, slowly adding the pre-prepared aqueous alkaline hydrogen peroxide solution dropwise ...
Embodiment 2
[0021] 1) After mixing p-cresol, acetic anhydride and concentrated sulfuric acid in a molar ratio of 1:2:0.1, reflux reaction under stirring for 8 hours, after the reaction, distill off the generated acetic acid and excess acetic anhydride at 250°C to obtain phenolic ester ;
[0022] 2) At 200°C, add anhydrous aluminum trichloride to the phenolic ester, continue stirring for 5 hours, add 4mol / L hydrochloric acid at 150°C for hydrolysis reaction, after the solution is separated, separate the upper organic layer , the lower aqueous layer was extracted with xylene, all organic layers were kept and combined, and concentrated under reduced pressure to obtain phenolic ketone 2-hydroxy-5-methylacetophenone, wherein the molar ratio of phenolic ester and aluminum chloride was 1:5 , the molar ratio of phenol ester and hydrochloric acid is 1:10.
[0023] 3) Dissolve 2-hydroxy-5-methylacetophenone in 2-propanol with 20 times the mass, slowly add the pre-prepared aqueous alkaline hydrogen...
Embodiment 3
[0026] In a 500ml three-necked flask equipped with a stirrer, a thermometer, and a detachable reflux condensing device, add 70g of p-cresol, then add 70g of acetic anhydride and 5 drops of concentrated sulfuric acid, heat in an oil bath, and reflux for 2 hours under stirring, then change to reflux The device is an atmospheric distillation device, the temperature of the oil bath is slowly raised to 200°C, the temperature of the kettle is 170°C, and the acetic acid and excess acetic anhydride generated by the reaction are distilled off. The temperature of the reaction liquid was adjusted and maintained at 110° C., and 120 g of anhydrous aluminum trichloride was added. After the addition is complete, heat and stir the reaction for 1 hour, stop stirring, slowly add 300mL of 4mol / L hydrochloric acid, keep the temperature of the reaction solution at 115°C, keep the flask in a boiling state, and slowly hydrolyze the solid until it disappears. During this process, there is a small amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com