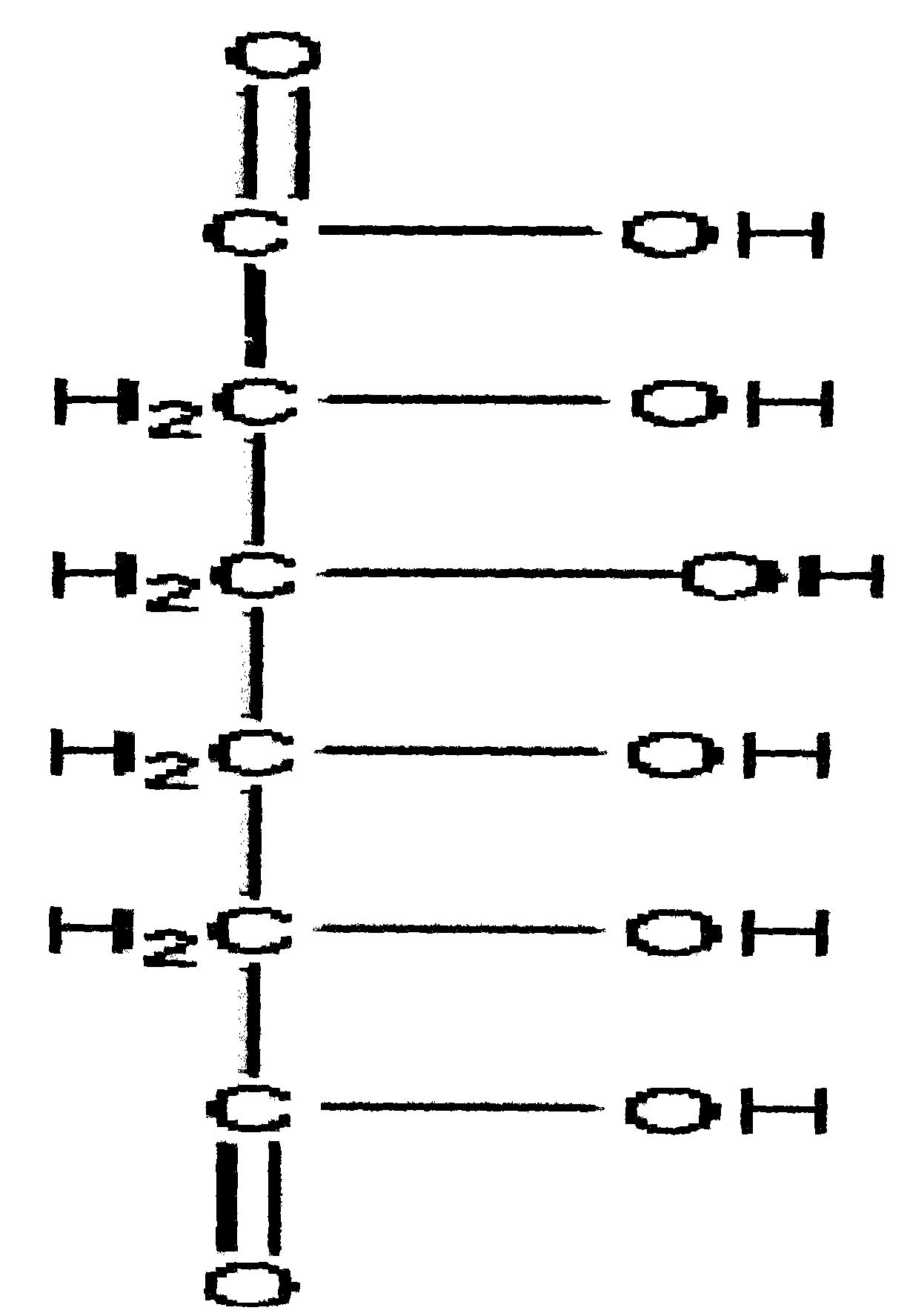
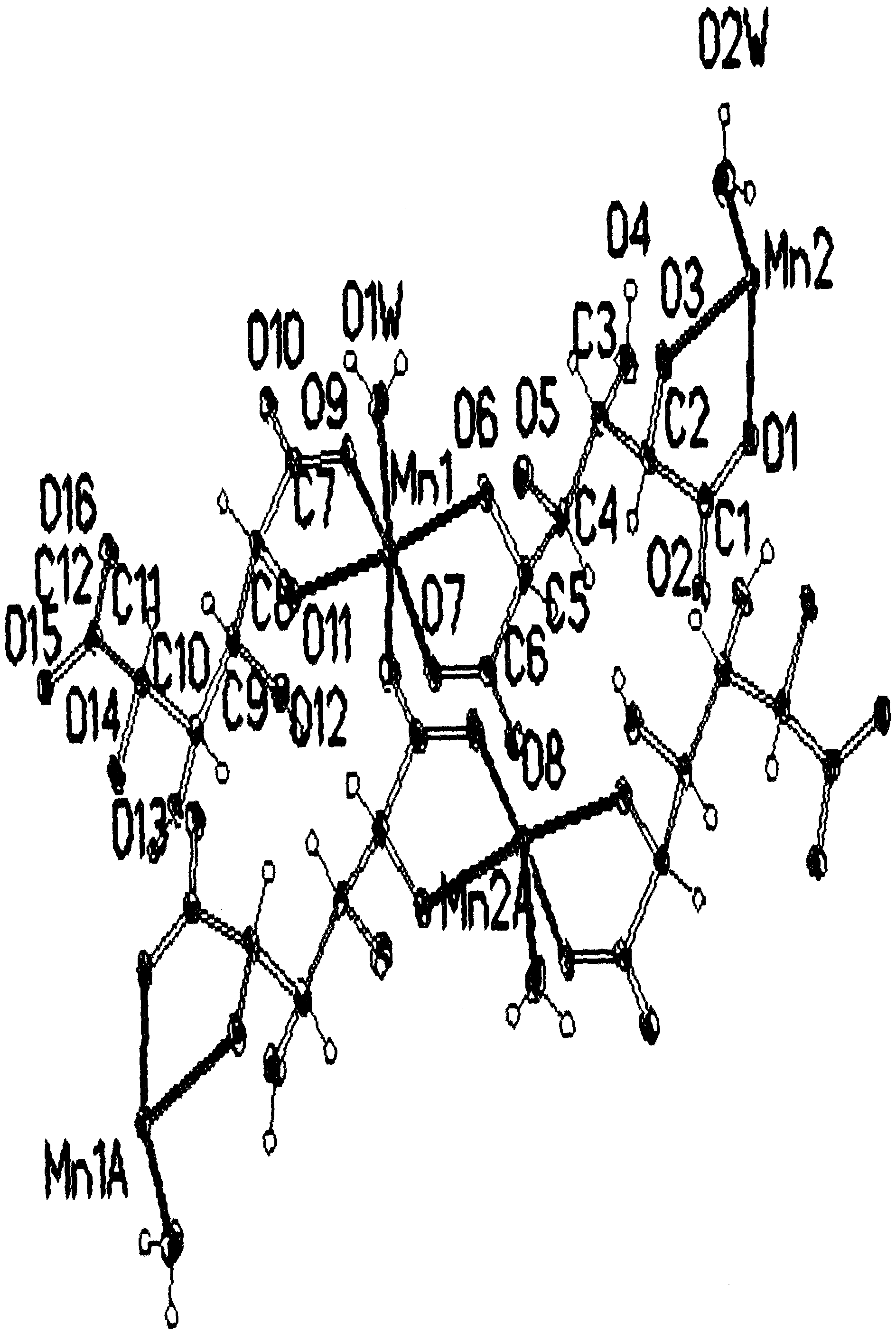
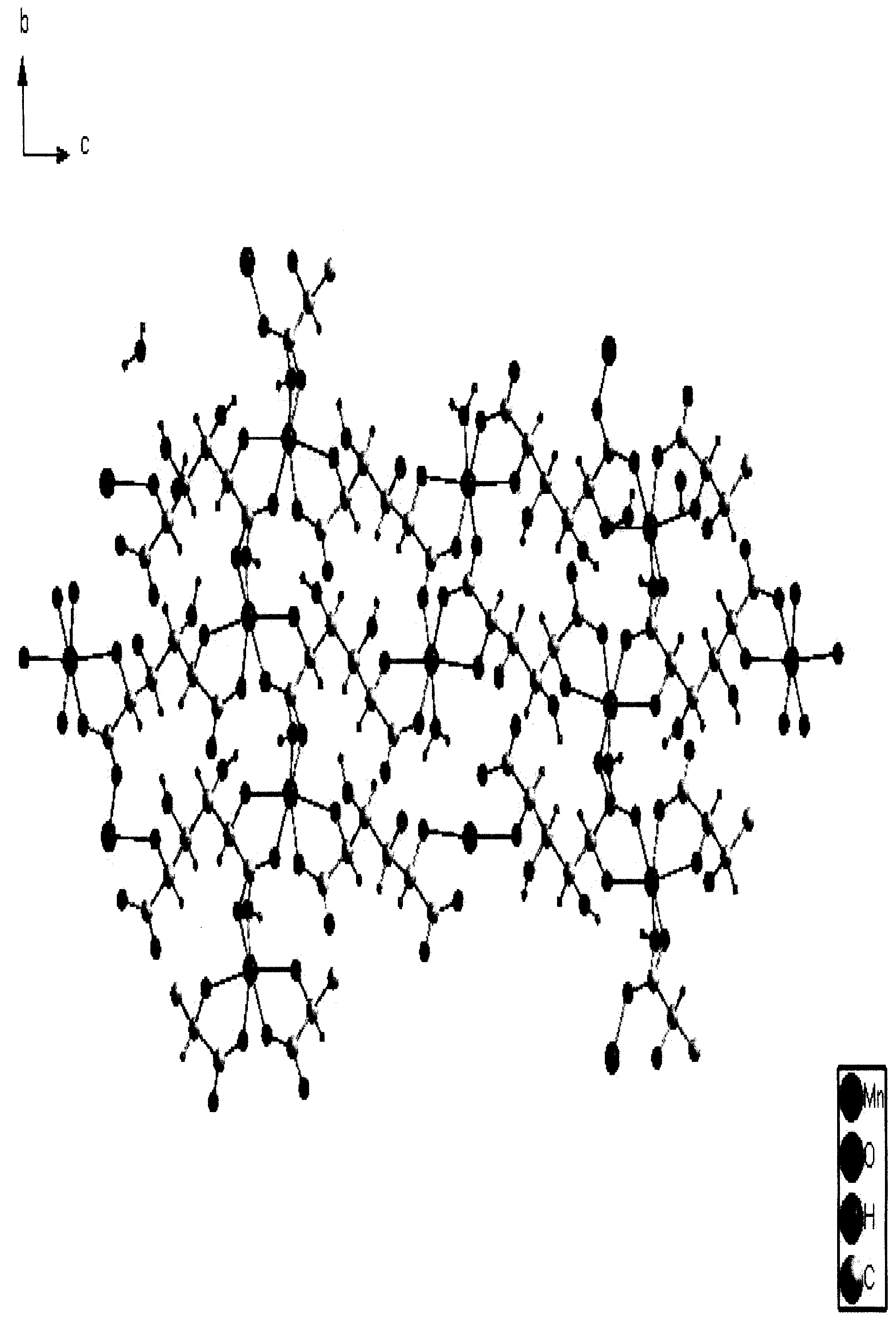
Nickel-hydrogen battery anode material prepared from glucaric acid metal complex doped beta-Ni(OH)2 and method thereof
A technology of glucaric acid and metal complexes, applied in battery electrodes, nickel oxide/nickel hydroxide, circuits, etc., can solve problems such as unsatisfactory, low depth of discharge, and reduced negative electrode performance, so as to improve charging efficiency and utilization efficiency and improve the cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] In this example, the cadmium gluconate complex is doped with β-Ni(OH) 2 The preparation process of the prepared Ni-MH battery cathode material is as follows:
[0049] 1. Selection of raw materials
[0050] (1) Choice of metal salt: choose cadmium acetate.
[0051] (2) The choice of organic solvent, select ethanol.
[0052] (3) Selection of alkaline solution: choose potassium hydroxide.
[0053] (4) Selection of conductive agent: select nickel (powder) and graphite (powder).
[0054] (5) Choice of binder: choose polytetrafluoroethylene and carboxymethyl cellulose.
[0055] 2. Preparation of Ni-MH battery cathode material
[0056] (1) Cadmium acetate and potassium gluconate were dissolved in an aqueous solution at a molar ratio of 1:1, heated and fully stirred, and reacted at 80° C. for 4 hours to obtain a white suspension of cadmium gluconate complex.
[0057] (2) Adding an appropriate amount of ethanol, lowering the temperature and cooling, standing for precipitat...
Embodiment 2
[0063] In this embodiment, manganese gluconate and yttrium gluconate complexes are doped with β-Ni(OH) 2 The preparation process of the nickel-metal hydride battery cathode material prepared is as follows
[0064] 1. Selection of raw materials
[0065] (1) Selection of metal salt: select manganese chloride and yttrium nitrate.
[0066] (2) The choice of organic solvent, select ethanol.
[0067] (3) Selection of alkaline solution: choose sodium hydroxide.
[0068] (4) Choice of conductive agent: choose acetylene black (powder).
[0069] (5) Choice of adhesive: choose polytetrafluoroethylene.
[0070] 2. Preparation of Ni-MH battery cathode material
[0071] (1) Dissolve manganese chloride, yttrium nitrate and potassium gluconate in an aqueous solution at a molar ratio of 0.9:0.1:1, heat and fully stir, and react at 80°C for 4 hours to obtain a white suspension of the glucaric acid metal complex liquid.
[0072] (2) Add an appropriate amount of ethanol, lower the temperat...
Embodiment 3
[0078] In this example, cobalt glucarate and zinc glucarate complexes are doped with β-Ni(OH) 2 The preparation process of the nickel-metal hydride battery cathode material prepared is as follows
[0079] 1. Selection of raw materials
[0080] (1) Selection of metal salt: select cobalt chloride and zinc chloride.
[0081] (2) Selection of organic solvent: select ethanol.
[0082] (3) Selection of alkaline solution: choose potassium hydroxide.
[0083] (4) Selection of conductive agent: select nickel (powder) and graphite (powder).
[0084] (5) Choice of binder: choose polytetrafluoroethylene and carboxymethyl cellulose.
[0085] 2. Preparation of Ni-MH battery cathode material
[0086] (1) Cobalt chloride, zinc chloride and potassium glucarate were dissolved in the aqueous solution at a molar ratio of 0.7:0.3:1, heated and fully stirred, and reacted at 80°C for 4 hours to obtain glucaric acid metal complex pink colored suspension.
[0087] (2) Add appropriate amount of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com