Organic molecular probe material with nucleus-shell structure and preparation method thereof
A technology of organic molecules and shell structures, applied in the direction of luminescent materials, material excitation analysis, chemical instruments and methods, etc., can solve the problems of background luminescence interference and weak penetrating power, achieve stable fluorescence luminescence performance, strengthen ligand exchange, Apply a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
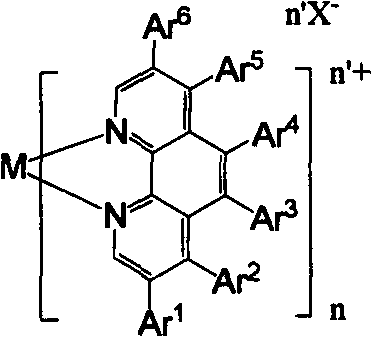
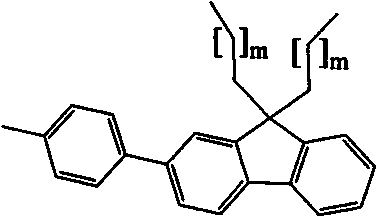
[0035] 1) Transition metal complex Re(phen)(CO) 3 Cl is the synthesis of 3-{2-[9,9-bis[6-(N-carbazolylhexyl)fluorenyl]]}-phenyl-phenanthroline tricarbonyl rhenium chloride:
[0036] 422 mg (0.5 mmol) and Re(CO) 3 Cl 181mg (0.5mmol) was added to 50mL toluene, refluxed for 12 hours, the solvent was evaporated, and then the residue was chromatographed on a silica gel column with dichloromethane as the elution meter to obtain the transition metal complex Re(phen )(CO) 3 Cl 390 mg, yield 68%. 1 H NMR (400MHz, CDCl 3 , TMS) δ = 9.59 (d, J = 1.6Hz, 1H), 9.40 (d, J = 4.4Hz, 1H), 8.52 (t, J = 3.4Hz, 2H), 8.03 (dd, J = 3.6Hz, 7.6Hz, 4H), 7.95(dd, J=8.8Hz, 15.2Hz, 2H), 7.86(d, J=7.6Hz, 1H), 7.83(t, J=8.0Hz, 1H), 7.76(d, J =7.2Hz, 1H), 7.68(d, J=8.0Hz, 1H), 7.64(s, 1H), 7.38(t, J=8.0Hz, 5H), 7.32(t, J=7.2Hz, 1H), 7.28(d, J=8.4Hz, 5H), 7.16(t, J=7.4Hz, 4H), 4.15(t, J=7.2Hz, 4H), 2.10-1.93(m, 4H), 1.68(t, J =6.4Hz, 4H), 1.20-1.12(m, 8H), 0.65-0.61(m, 4H), 13 C NMR (75MHz, CDCl 3, T...
Embodiment 2
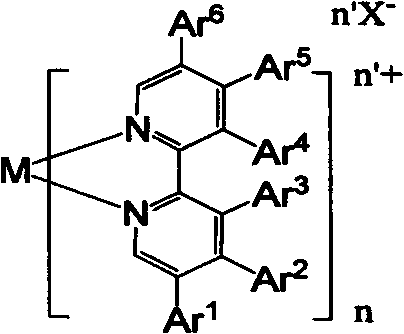
[0042] 1) Transition metal complex Ru(bpy) 3 (PF 6 ) 2 Namely three {5-[2-[9,9-bis[6-(N-carbazolylhexyl)fluorenyl]]]-phenyl-2,2'-bipyridyl} divalent ruthenium hexafluorophosphate Synthesis of salt:
[0043] 169.5 mg (0.3 mmol) of 5-[2-[9,9-bis[6-(N-carbazolylhexyl)fluorenyl]]]-phenyl-2,2'-bipyridine and [Ru(DMSO ) 4 ]Cl 2 Add 48.5mg (0.1mmol) into 20mL ethanol solution, reflux for 24 hours under dark conditions; then add 80mg KPF 6 (80 mg) continued to reflux for 2 hours; after cooling to room temperature, 30 milliliters of water was added, the precipitated product was filtered out, and washed twice with 5 milliliters of water; then silica gel column chromatography was used to elute the product with dichloromethane containing 3% acetone , after vacuum drying, 104 mg of transition metal complex Ru(bpy) in the form of red solid powder can be obtained 3 (PF 6 ) 2 . MS (MALDI-ToF): m / z 1941.2. 1 H NMR (500MHz, CDCl3): 8.62 (bs, 3H, PyH), 8.55 (bs, 3H, PyH), 8.01 (bs, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com