Novel ketolide compound and preparation method and application thereof
A compound and ketolide technology, which is applied in the field of telithromycin ketolide-like compounds with aromatic ring-linked tetrazolylidines as side chains and the preparation thereof, can solve problems such as rising, and achieve strong activity and major industrialization prospects , the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
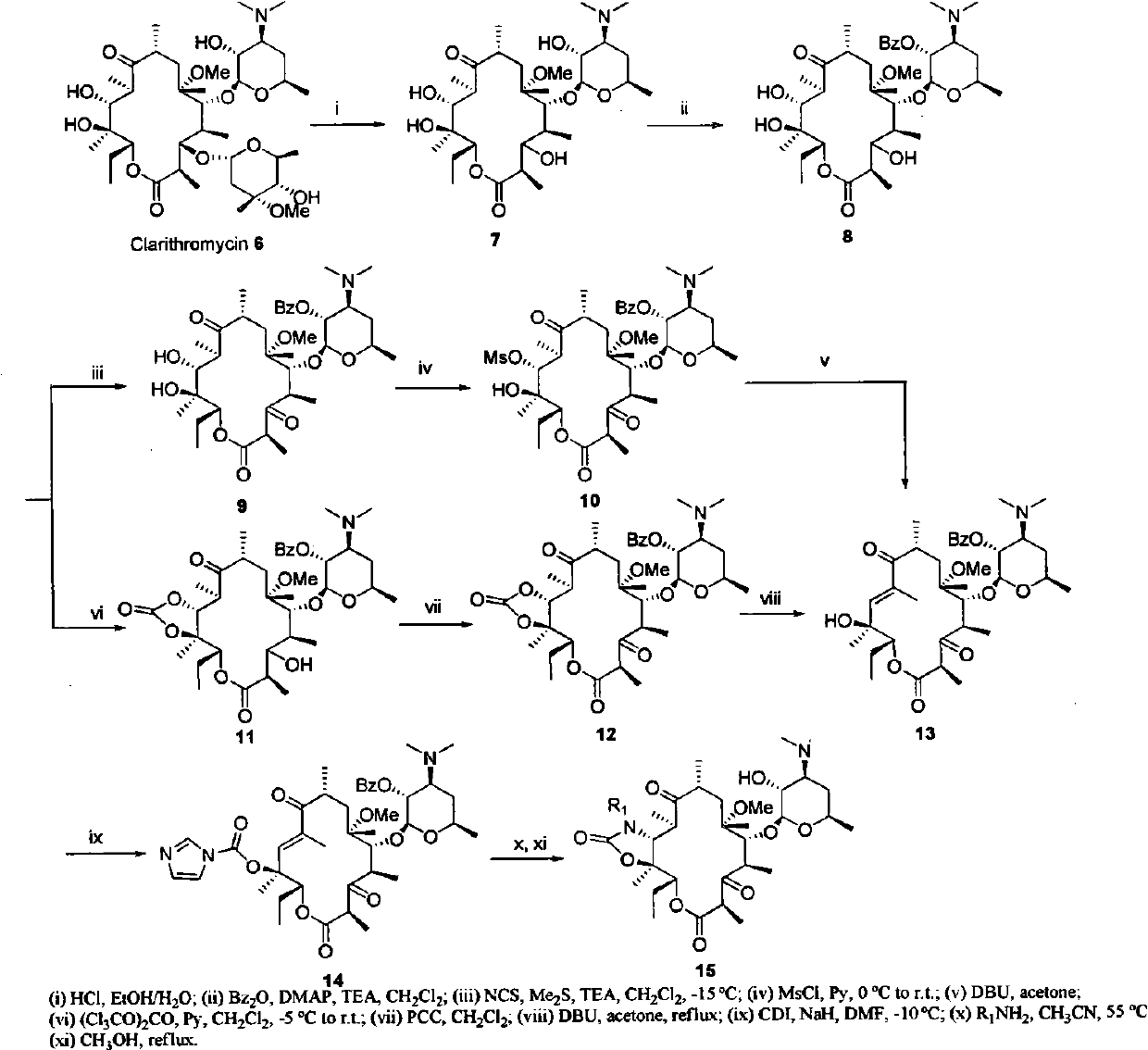
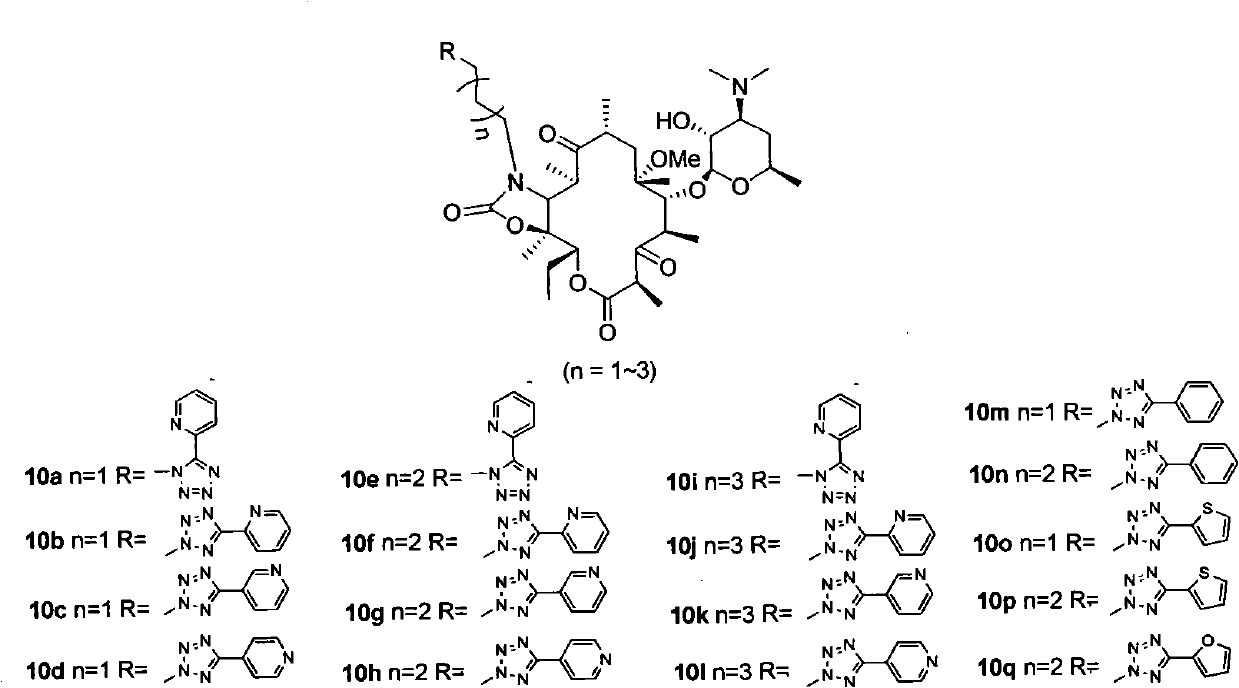
Image
Examples
Embodiment 1
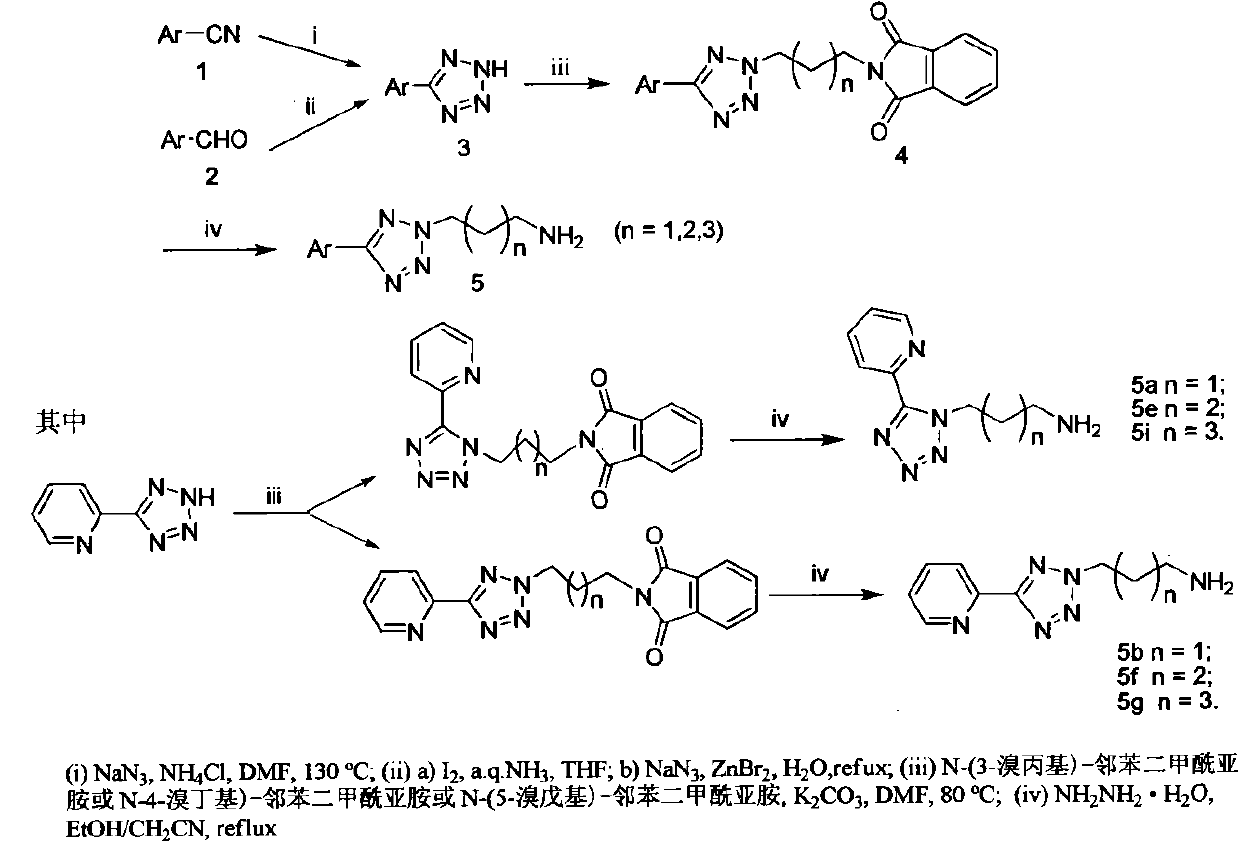
[0040] Preparation of Aromatic Tetrazoles
[0041] method one:
[0042] Add cyanopyridine (1,1 mmol) into a three-necked flask and dissolve it in N,N-dimethylformamide (1.25mL), add sodium azide (3equiv) and ammonium chloride (3equiv) in sequence under stirring ; Under nitrogen protection, react at 130°C in an oil bath for 22h; cool to room temperature, add saturated sodium bicarbonate solution (2.5mL) and ethyl acetate (2.5mL) to the reaction solution, shake, transfer to a separatory funnel, and separate The organic layer; the solution layer was slowly acidified with concentrated hydrochloric acid to pH 4-5, stirred and left to stand, and crystals were precipitated; suction filtered, the mother liquor was cooled in ice water, and crystals continued to be precipitated, and the above crystals were combined, and an appropriate amount of The product was washed with distilled water to obtain the product. When the aryl nitrile is 2-cyanopyridine, the product 3a is obtained; when ...
Embodiment 2
[0052] The product 3 (1 mmol) obtained in Example 1 was dissolved in N,N-dimethylformamide (5 mL), and potassium carbonate (1 mmol) and N-bromoalkylphthalimide (1 mmol) were added in sequence; Under the protection of nitrogen, react at 80°C for 10h. After the reaction is complete, pour the reaction solution into 10 mL of ice water, filter, wash the filter cake with a small amount of ice water, and use petroleum ether: ethyl acetate = 1:1 to petroleum ether: ethyl acetate: dichloromethane = 1:1: 1 is developing agent silica gel (200-300 order) column chromatography, obtains the product. Depending on the starting material 3, different products 4a-4q can be obtained.
[0053] 4a: 2-[3-(5-(Pyridin-2-yl)-1H-tetrazol-1-yl)propyl)]-isoindole-1,3-2H-dione, white solid, yield rate 35%, 1 H NMR (300MHz, CDCl 3 ): δ8.83(m, 3H), 8.33(d, J=7.8Hz, 1H), 7.83(m, 1H), 7.72(m, 2H), 7.35(m, 2H), 5.03(t, J= 7.8Hz, 2H), 3.83(t, J=6.9Hz, 2H), 2.39(m, 2H); 13 C NMR (CDCl 3 ): δ168.1, 151.5, 1...
Embodiment 3
[0071]Dissolve the product 4 (1 mmol) obtained in Example 2 in a mixture of absolute ethanol (7 mL) and acetonitrile (5 mL), add hydrazine monohydrate (2 equiv), and heat to reflux for 6 h; after the reaction is complete, cool to room temperature; Filter the reaction solution, wash the filter cake with a small amount of 95% ethanol, remove the solvent in the filtrate by rotary evaporation, add sodium hydroxide solution (2mol / L, 10mL) to the residue; add dichloromethane (10mL×3) for extraction, and combine the organic phases , washed with saturated brine, and dried over anhydrous sodium sulfate. Filtrate, concentrate, and use dichloromethane:methanol:triethylamine=9:1:0.1 as the developing solvent for silica gel (200-300 mesh) column chromatography to obtain the aromatic ring tetrazolidinyl amine product. According to the different raw materials 4a-4q, the products 5a-5q can be correspondingly obtained respectively.
[0072] 5a: 3-[5-(pyridin-2-yl)-1H-tetrazol-1-yl]propylamine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com