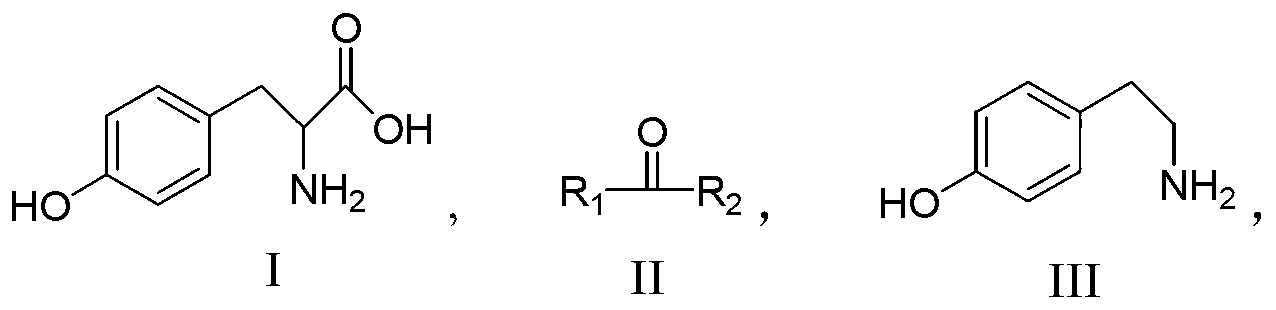
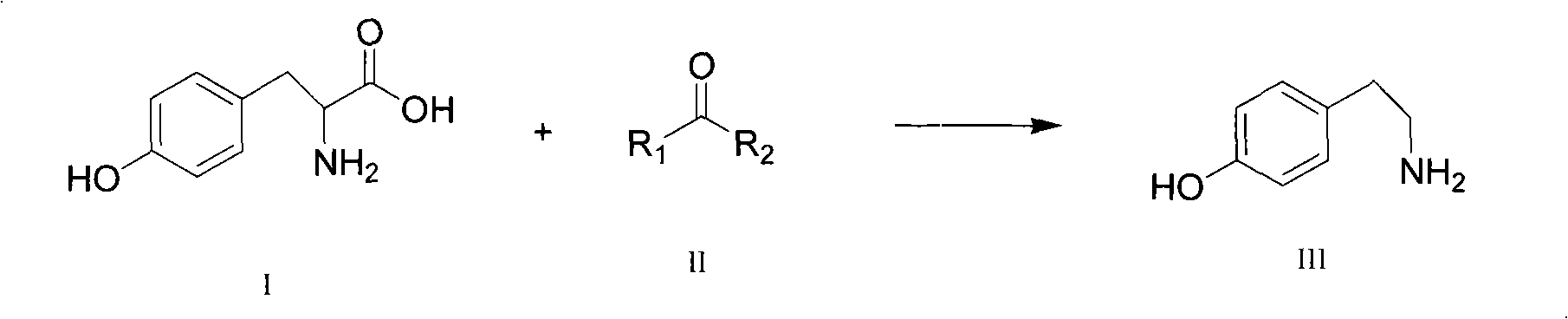
New method for preparing tyramine
A technology for tyramide and decarboxylation reaction, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems of unsuitability for industrial production, poor product quality, low reaction yield, etc., and achieves easy industrialization. The effect of production, good quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 10 g of tyrosine, 6 g of diisoamyl ketone, and 20 g of cyclohexanol into a 100 mL reaction flask, pass through nitrogen to protect and raise the temperature to reflux (the temperature is between 140 and 150° C.), and remove the generated water in time. React until the solution turns into a brownish-red transparent liquid (about 4-6 hours). After cooling the reaction liquid, add 25 g of water, stir overnight with nitrogen gas, add 8 g of ether, stir, filter with suction, and rinse with 6 g of methanol to obtain the product. Vacuum dried to obtain 4.9 g of white tyramide product, content: 98.4%. Yield 64.4%. MP: 161-162°C (Waser, E. Phenylalanine series. VI. Decarboxylation of tryosine andleucine. Helvetica Chimica Acta (1925), 8758-73, 164-165°C). MS(EI): m / e=137; IR(KBr)cm -1 : 3330, 3300, 1600, 1520, 1260, 820. It is completely consistent with the Sadtler standard spectrum (spectrum number 18175K); 1 HNMR (500MHZ, CDCl3): δppm: 3.3-3.5 (4H, -CH2-CH2-, t); 7.4-7....
Embodiment 2
[0032] Add 10 g of tyrosine and 20 g of diisoamyl ketone into a 100 mL reaction flask, pass through nitrogen to protect and raise the temperature (the temperature is between 160 and 170° C.), and remove the generated water in time. React until the solution turns into a brown-red transparent liquid (about 3 to 5 hours). After cooling the reaction liquid, add 25 g of water, stir overnight with nitrogen gas, add 8 g of ether, stir, filter with suction, and rinse with 6 g of methanol to obtain the product. Vacuum dried to obtain 5.5 g of white tyramide product, content: 98.1%. Yield 68.4%. MP: 162-164°C.
Embodiment 3
[0034]Add 10g of tyrosine, 13g of cyclohexanol, and 5g of acetophenone into a 100mL reaction flask, pass through nitrogen to protect and raise the temperature to reflux (the temperature is between 160-170°C), and remove the generated water in time. React until the solution turns into a brownish-red transparent liquid (about 2 to 3 hours). After cooling the reaction liquid, add 25 g of water, stir overnight with nitrogen, add 8 g of ether, stir, filter with suction, and rinse with 6 g of methanol to obtain the product. Vacuum dried to obtain 5.2 g of white tyramide product, content: 99.4%. Yield 72.3%. MP: 163-164°C.
[0035] The present invention adopts the decarboxylation process of ketone-catalyzed amino acid, which greatly reduces the decarboxylation temperature from 255-260 to 140-170 degrees, improves the yield of the product, and avoids the problems of denaturation and color depth of the product at high temperature. After simple washing, a white high-purity product tyr...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap