Anode material for lithium ion battery and preparation method thereof
A technology for lithium ion batteries and negative electrode materials, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of poor cycle performance and low capacity, and achieve the effects of good cycle performance, alleviation of volume changes, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] At normal pressure and in the temperature range of 0-5°C, Fe(NO 3 ) 3 Uniformly disperse in pyrrole, then slowly add oxidant hydrogen peroxide (H 2 o 2 ). where Fe(NO 3 ) 3 The molar ratio to the oxidant is 1:3, Fe(NO 3 ) 3 The molar ratio to pyrrole is 1:1. After fully stirring and reacting for 8 hours, the complex (black solid) of Fe and polypyrrole was obtained through filtration, washing and drying.
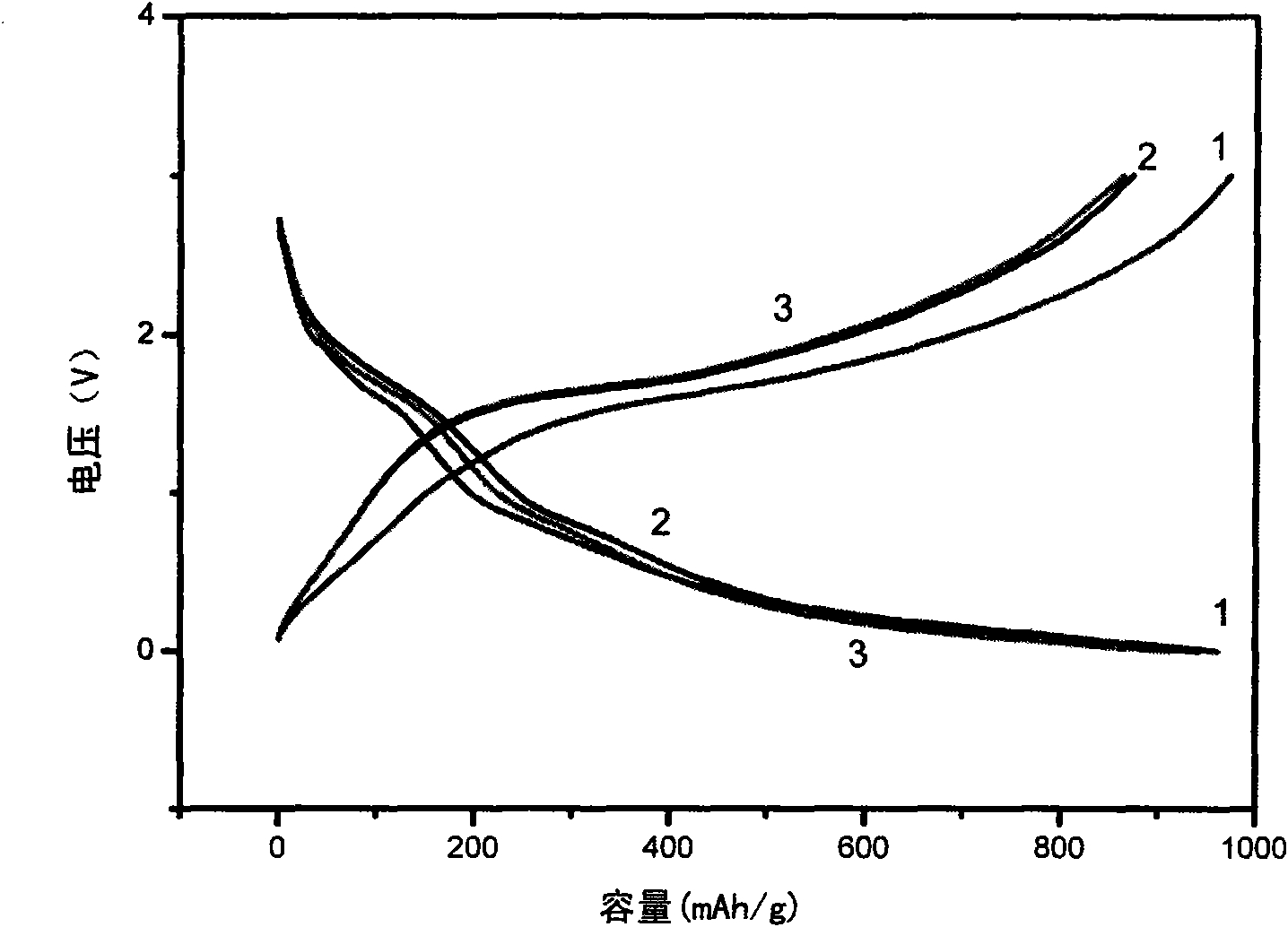
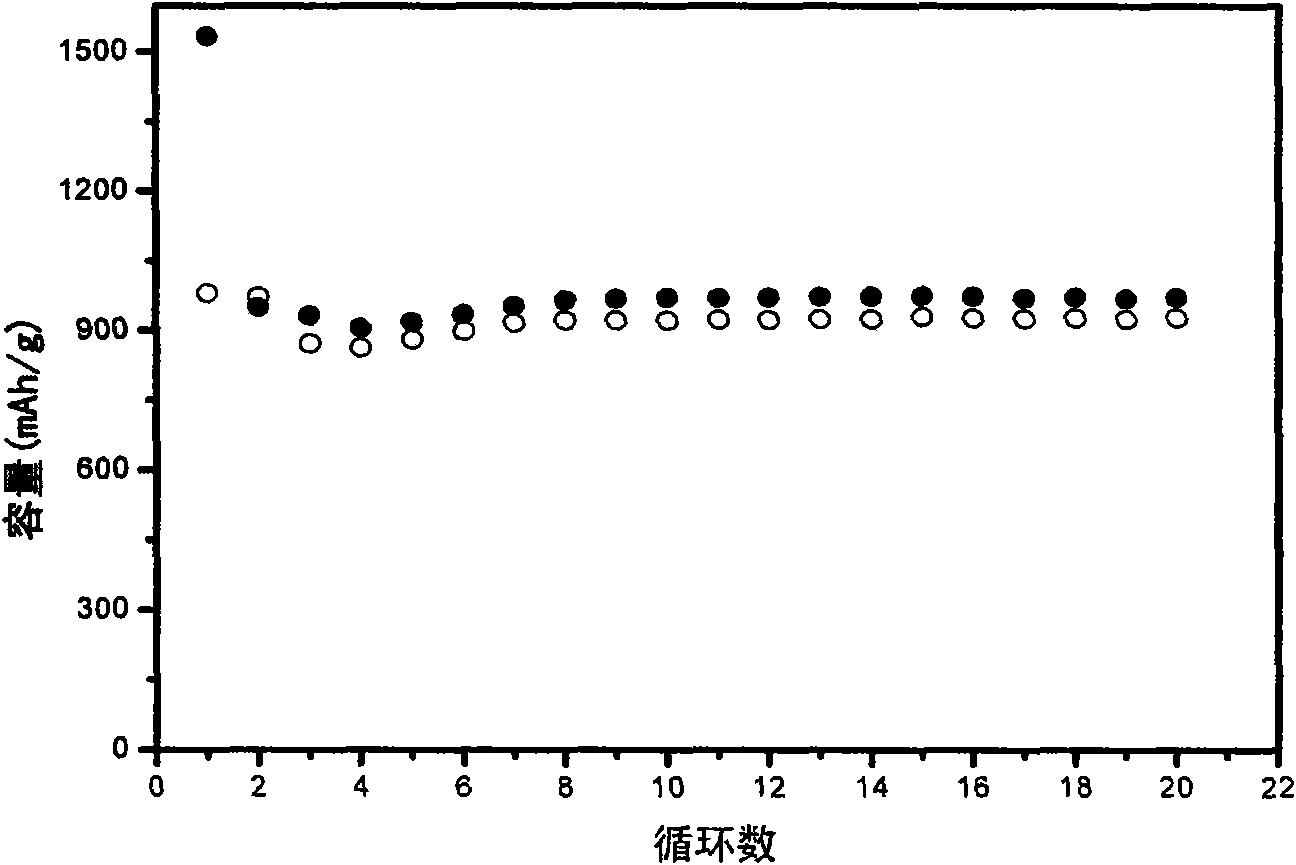
[0031]The lithium storage performance of the Fe / polypyrrole complex was investigated by a button-type simulated battery. The Fe / polypyrrole complex was used as the working electrode of the coin cell. The preparation method of the working electrode sheet is as follows: under normal temperature and pressure, the complex powder is uniformly mixed with the N-methylpyrrolidone (NMP) solution of conductive carbon black and polyvinylidene fluoride (PVDF) (the weight ratio of the three after drying is 80:10:10), the slurry is prepared and evenly coated on the copper ...
Embodiment 2
[0036] Add Co(NO 3 ) 2 And evenly disperse, then add the oxidant hydrogen peroxide (H 2 o 2 ). After fully reacting, filtering, washing and drying, a black Co / polypyrrole complex solid powder is obtained.
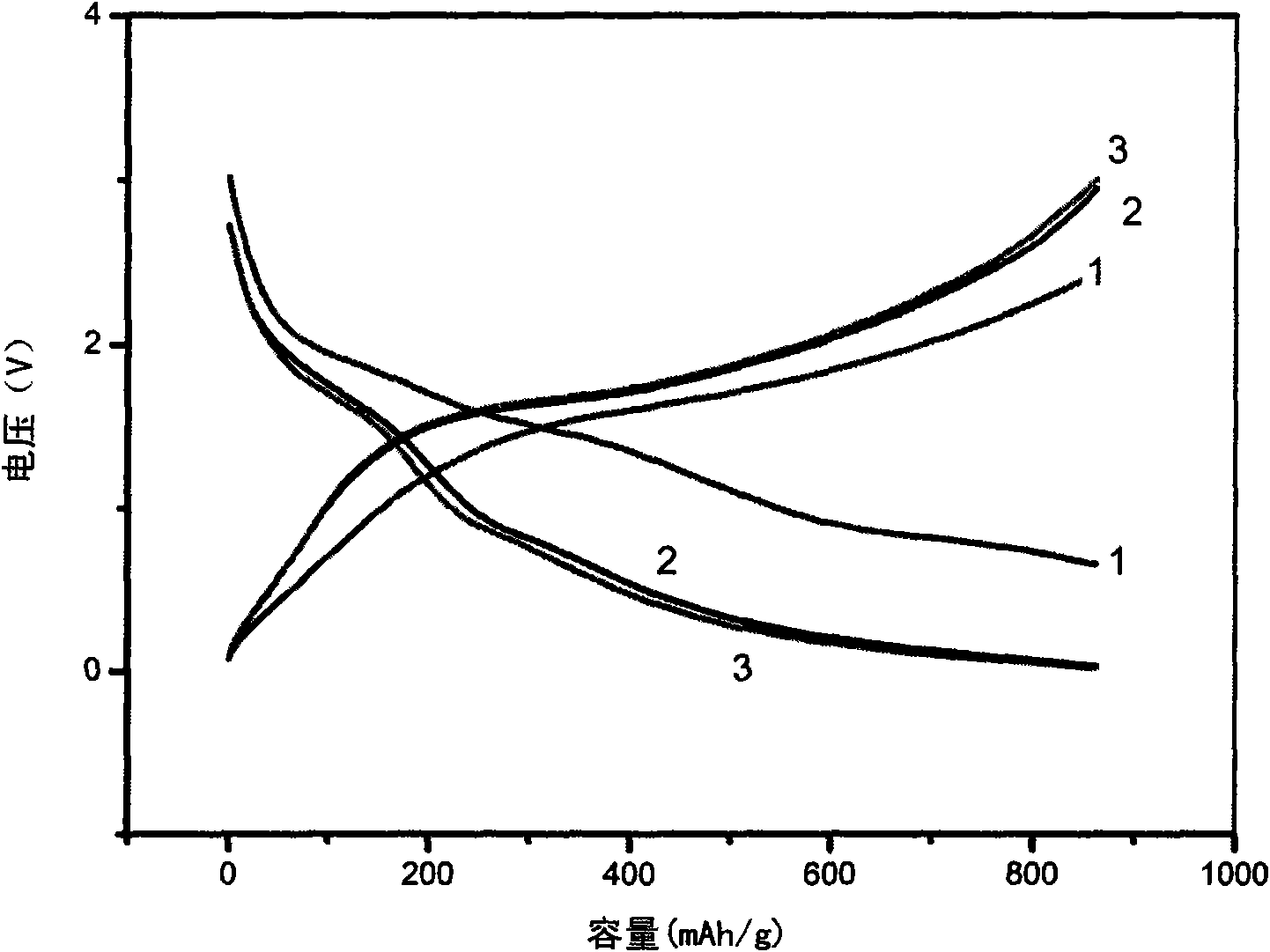
[0037] The preparation of the working electrode of the button-type simulated battery, the assembly process and conditions of the battery and the test method are the same as in Example 1, and the charge-discharge curve of the simulated battery is as follows: image 3 shown. from image 3 It can be seen that the complex of Co and polypyrrole formed by coordination has a high capacity, and the coordination with metal cobalt makes polypyrrole electrochemically active.
Embodiment 3
[0039] Add CuCl to liquid pyridine 2 , after uniform dispersion, the oxidant iron trichloride (FeCl 3 ). After fully reacting, after filtering, washing and drying, a black solid is obtained, which is the complex of Cu and pyridine.
[0040] The preparation of the working electrode of simulation battery, the assembly of battery and test method are the same as example 1, and the charge-discharge curve of simulation battery is as follows Figure 4 shown. from Figure 4 It can be seen that the complexes of Cu and pyridine formed by coordination have higher capacity, among which pyridine is electrochemically active.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com