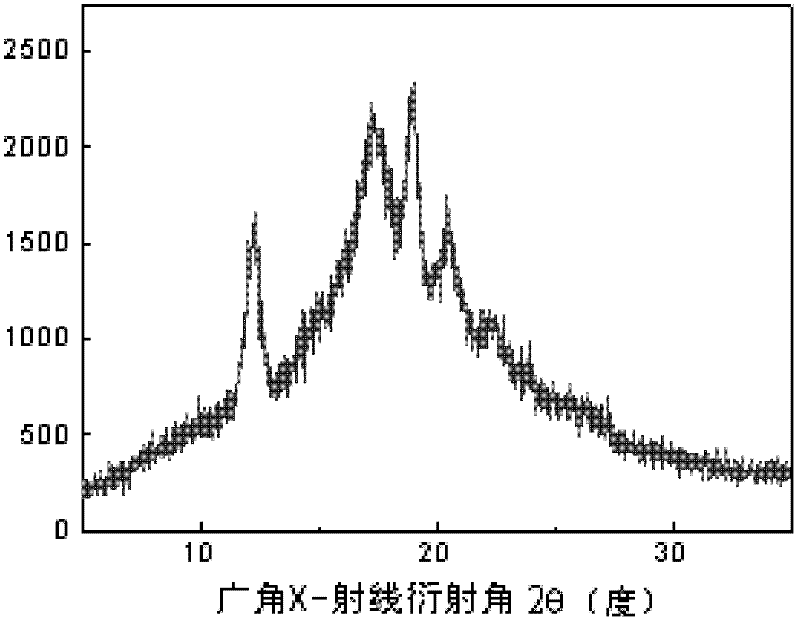
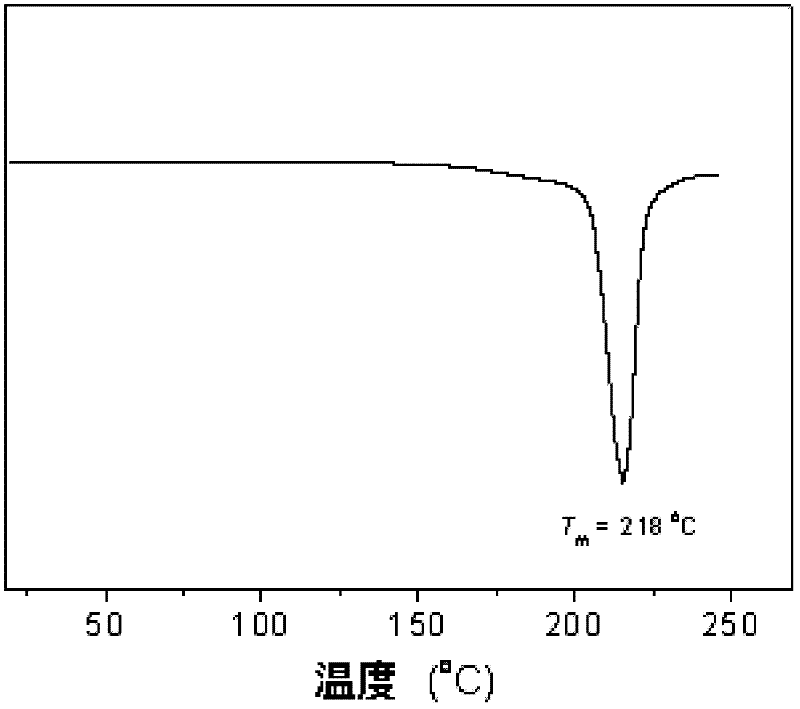
Crystallizable polycarbonate material based on carbon dioxide and preparation method thereof
A polycarbonate and carbon dioxide technology, which is applied in the field of polymer materials, can solve the problems of thermal stability of polymer products, poor mechanical properties and transparency, difficult polymer processing, and inability to crystallize, and achieves obvious crystallization effect, improved transparency, and polymerization. The effect of high material regularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add in the stainless steel autoclave with an effective volume of 200mL and the ambient temperature in the following order: 0.5×10 -3 Mole (R, R)-SalenCoX(R 1 = T-Bu, R 2 =1-adamantyl, R 3 = T-Bu, R 4 = T-Bu, R 5 , R 6 Yes-(CH 2 ) 4 -, X is acetate anion), 0.5×10 -3 Moles of bis-(triphenylphosphonium) ammonium chloride, 0.25 moles of epoxy cyclohexane, 0.25 moles of (R)-2-methyltetrahydrofuran, control the temperature at -10°C, and then pass in carbon dioxide gas and maintain 0.8MPa After reacting at a constant pressure for 24 hours under magnetic stirring, the unreacted carbon dioxide in the autoclave was slowly released, and a large amount of ether was added to precipitate the polycarbonate. It was filtered, washed with ether for several times, and dried in vacuum to constant weight to obtain 20 g of polycyclohexenyl carbonate white solid. The average molecular weight of the polymer determined by gel permeation chromatography was 18000, and the molecular weight distribut...
Embodiment 2
[0036] Using the same equipment in Example 1, under the same conditions, but after the temperature was lowered to -25°C and reacted for 48 hours, 14 grams of polycyclohexenyl carbonate was obtained, with a molecular weight of 12,000 and a molecular weight distribution of 1.15. The content of carbonate units in the product reaches 99%. by 13 C-NMR analysis found that the polymer had an identical structure exceeding 98%. After a small amount of hydrolyzed polycarbonate was analyzed by chiral gas phase column, it was found that the enantioselectivity of 1,2-cyclohexanediol was 96% (SS / RR= 98 / 2); After the obtained polymer was kept at a constant temperature of 180°C for 5 minutes, and analyzed by DSC, it was found that the glass transition temperature of the polymer was 118°C, the maximum crystal melting peak appeared at 218°C, and the enthalpy of fusion was 27.1 J / g; Thermogravimetric analysis shows that its 50% material decomposition temperature is 310℃.
Embodiment 3
[0038] Using the same equipment in Example 1, under the same conditions, only 0.25 mol of (S)-2-methyltetrahydrofuran was increased to 1.0 mol, and after reacting at -10°C for 24 hours, 12 g of polycyclohexane carbonate was obtained. The olefin ester has a molecular weight of 11,000, a molecular weight distribution of 1.15, and the content of carbonate units in the polymer reaches 99%. by 13 C-NMR analysis found that the polymer had an identical structure exceeding 90%. After a small amount of hydrolyzed polycarbonate was analyzed by a chiral gas phase column, it was found that the enantioselectivity of 1,2-cyclohexanediol was 90% (SS / RR= 95 / 5); After keeping the obtained polymer at a constant temperature of 180°C for 15 minutes, DSC analysis showed that the glass transition temperature of the polymer was 118°C, the maximum crystal melting peak appeared at 217°C, and the melting enthalpy was 13.1 J / g; Thermogravimetric analysis shows that its 50% material decomposition temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com