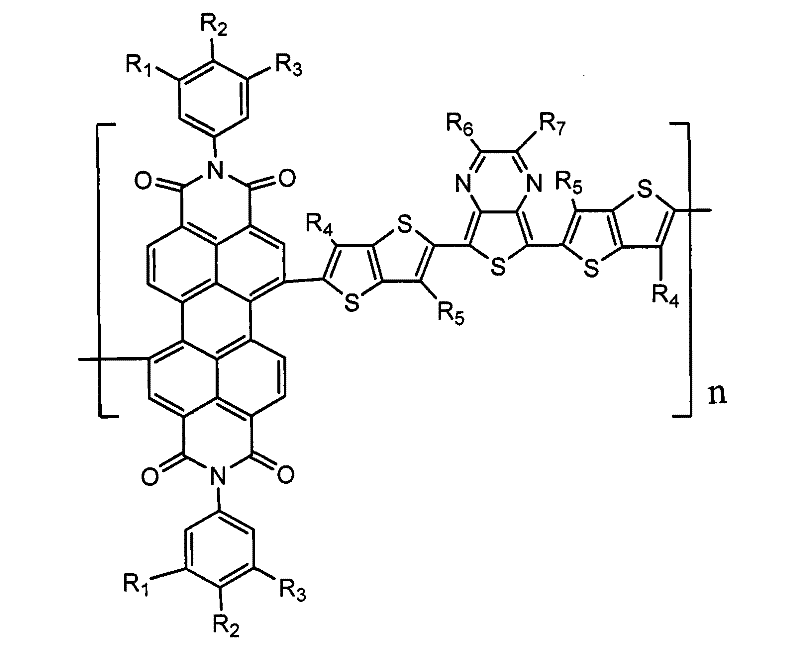
Thiophene-containing perylene tetracarboxylic diimide copolymer, and preparation method and application thereof
A perylenetetracarboxylic acid diimide and thiophene unit technology, which is applied in the field of organic compound synthesis, can solve the problems of reducing the photoelectric conversion efficiency of organic solar cells, inability to effectively use sunlight, and insufficient matching of emission spectra, and achieve technological Simple, excellent solubility and charge transport properties, and the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
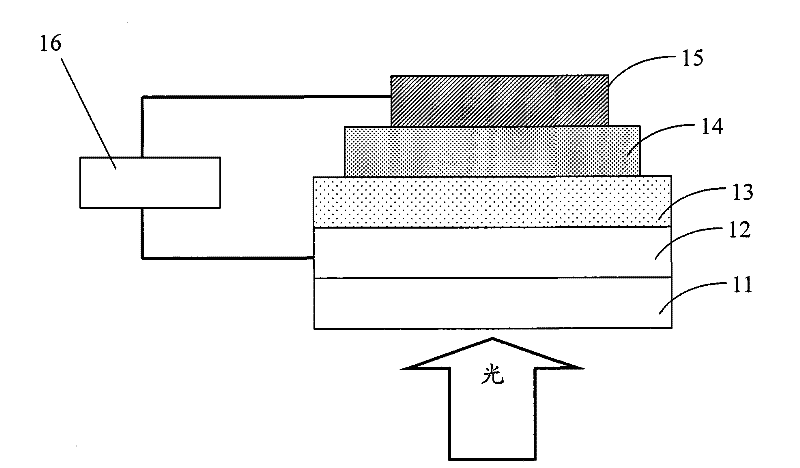
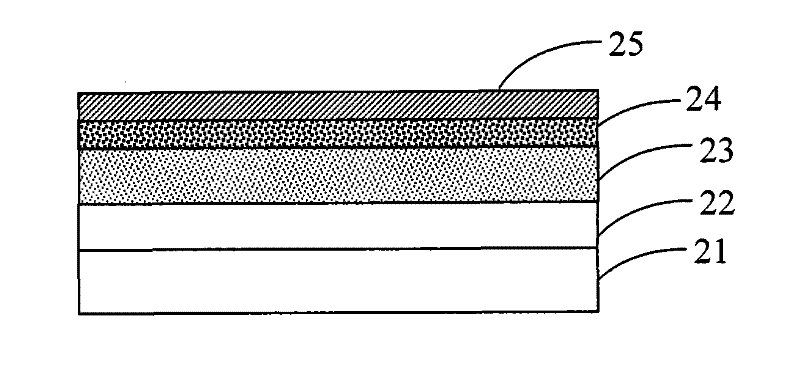
Image
Examples
preparation example Construction
[0036] And, this embodiment also provides the preparation method of the perylene tetracarboxylic diimide copolymer containing thiophene units, comprising the following steps:
[0037] 1.1 respectively provide the compound I represented by the following structural formula 1 , I 2 ,
[0038]
[0039] Among them, R 1 , R 2 , R 3 are the same or different groups selected from -H, C 1 ~C 15 Alkyl, C 1 ~C 15 Alkoxybenzene or phenyl, R 4 , R 5 are the same or different groups selected from C 1 ~C 15 Alkyl, C 1 ~C 15 Alkoxy, C 1 ~C 15 Alkoxybenzene or phenyl, R 6 , R 7 are the same or different -H, or selected from C 1 ~C 15 Alkyl, C 1 ~C 15 Alkoxy, C 1 ~C 15 Alkoxybenzene, phenyl;
[0040] 1.2 In a system containing a catalyst and an organic solvent, compound I 1 , I 2 Carry out Stille coupling reaction, generate the described thiophene unit perylenetetracarboxylic acid diimide copolymer containing thiophene unit of following formula compound I,
[0041]...
Embodiment 1
[0074] Poly N,N'-bis-(3,4,5-tri-dodecyloxybenzene)-3,4,9,10-perylenediimide-5,7-bis(3,6-bis The preparation of octylthieno[3,2-b]thiophene)-thieno[3,4-b]pyrazine, its structural formula is as follows:
[0075]
[0076] Its preparation comprises the following steps:
[0077] 1) Preparation of N, N'-two-(3,4,5-tri-dodecyloxybenzene)-1,7-dibromo-3,4,9,10-perylene diimide, which The reaction is shown in the following formula:
[0078]
[0079] The specific preparation process is: weigh 0.27mmol of 1,7-dibromo-3,4,9,10-perylenetetraanhydride in a reaction flask, add 0.84mmol of 3,4,5-tri-dodecyloxy- 1-Aminobenzene and 12ml of propionic acid were placed in ultrasonic for 10 minutes, then heated to 80°C after 30 minutes of nitrogen gas, and reacted for 48 hours; after the reaction was completed, cooled to room temperature, dissolved in chloroform, and then used Wash the organic layer with sodium bicarbonate solution to obtain a red suspension, filter, add anhydrous magnesium...
Embodiment 2
[0105] Poly N,N'-bis-(3,4,5-tri-octyloxybenzene)-3,4,9,10-perylenediimide-5,7-bis(3,6-dioctyl The preparation of thieno[3,2-b]thiophene)-2,3-dihexylthieno[3,4-b]pyrazine, its structural formula is as follows:
[0106]
[0107] Its preparation comprises the following steps:
[0108] 1) N, N'-bis-(3,4,5-tri-octyloxybenzene)-1,7-dibromo-3,4,9,10-perylene diimide was prepared as in Example 1 Middle (1) step;
[0109] 2) The preparation of 5,7-bis(5-tributyltin-thieno[3,2-b]thiophene))-2,3-dihexylthieno[3,4-b]pyrazine is as in Example 1 (2) to (8) steps;
[0110] 3) Poly N, N'-bis-(3,4,5-tri-octyloxybenzene)-3,4,9,10-perylenediimide-5,7-bis(3,6-di The preparation of octylthieno[3,2-b]thiophene)-2,3-dihexylthieno[3,4-b]pyrazine, its reaction is shown in the following formula:
[0111]
[0112] The specific preparation process is: under the protection of nitrogen, to the compound N, N'-bis-(3,4,5-tri-octyloxybenzene)-1,7-dibromo-3,4,9,10- Perylene diimide 0.5mmol, 5,7-bis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com