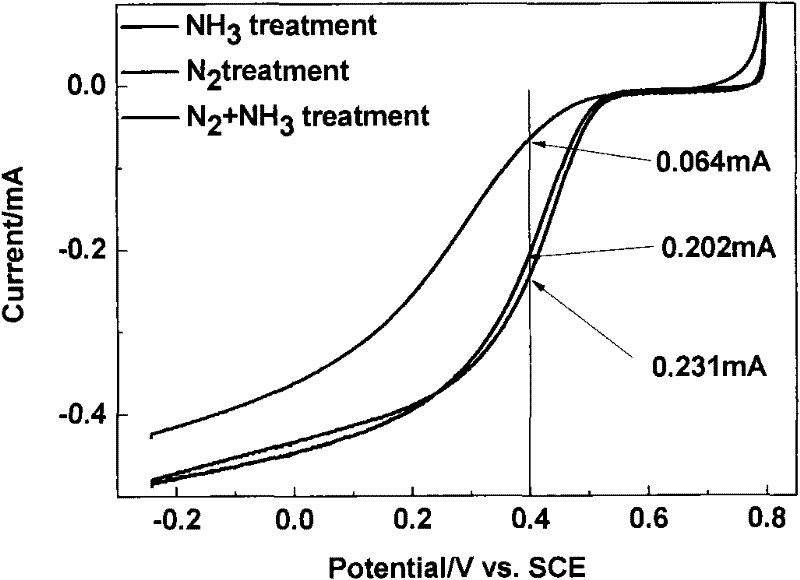
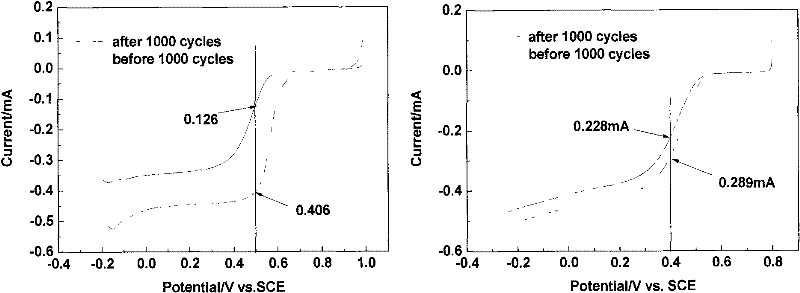
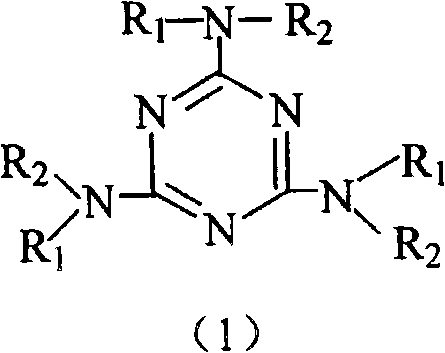
Carbon gel catalyst, its preparation and its application
A catalyst and carbon gel technology, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the limitations of large-scale industrial production, large proportion of airgel macropores, poor structural adjustability, etc. problems, to achieve the effect suitable for large-scale production, strong catalytic ability, and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Dissolve 3.53g of melamine in 4mL of deionized water to form solution A; add dropwise 6.3ml of formaldehyde solution with a mass concentration of 37% to solution A being stirred, stir at 60°C to form transparent solution B, and add to solution B Add ammonia water with a mass concentration of 2.8% dropwise, adjust the pH value to about 8, and continue to stir to obtain solution C. Take 0.4074g of cobalt nitrate solid and add it to the above-mentioned transparent solution C, mix well to obtain solution D; further stir until the reaction forms a gel E; transfer the gel E to a vacuum drying oven for 3 days of vacuum drying and aging treatment at 80°C, take it out and grind it to obtain solid powder F; carbonize the solid powder F at 800°C in a nitrogen atmosphere for 1h, N 2 Gas purged to room temperature, and then at 800 °C NH 3 Nitriding treatment in the atmosphere for 2h, to obtain solid powder G, with 2M HNO 3 The metal was washed out of the solution to obtain solid po...
Embodiment 2
[0057] Dissolve 3.53 g of melamine in 0.224 mol of formaldehyde solution with a mass concentration of 37%, stir at 50°C to form a transparent solution A, add NaOH solution dropwise to solution A, adjust the pH to 9, and continue stirring to obtain solution B , add 0.5656g of ferric nitrate solid to the above transparent solution B, mix well to obtain solution C; further stir until the reaction forms gel D; transfer gel D to a vacuum drying oven for 5 days of vacuum drying and aging treatment at 60°C, take out After crushing and grinding, the solid powder E was obtained; the solid powder E was carbonized in an argon atmosphere at 900°C for 3 hours, and the Ar gas was purged to room temperature, and then NH at 900°C 3 Nitriding treatment in the atmosphere for 5h, to obtain solid powder F, with 0.5M H 2 SO 4 The metal is washed out of the solution to obtain a solid powder G, that is, a melamine-iron-nitrogen carbon xerogel catalyst M-Fe-CN-20.
Embodiment 3
[0059] Dissolve 7.06g of melamine in a 0.448mol mass concentration of 37% formaldehyde solution, stir at 60°C to form a transparent solution A, and add Na 2 CO 3 Aqueous solution, adjust the pH value to 8, and continue to stir to obtain solution B. Take 0.339g of ammonium molybdate solid and add it to the above-mentioned transparent solution B, mix well to obtain solution C; further stir until the reaction forms gel D; transfer gel D Dry and age in a vacuum oven at 85°C for 7d, take it out, crush and grind to obtain solid powder E; carbonize solid powder E at 800°C in a nitrogen atmosphere for 2h, N 2 Gas purged to room temperature, then at 800 °C CH 3 Nitriding treatment in CN atmosphere for 2h, to obtain solid powder F, wash away the metal with 1M HCl solution, to obtain solid powder G, that is, melamine-molybdenum-nitrogen carbon xerogel catalyst M-Mo-CN-23.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com