Preparation method of monoclinic-phase lead tungstate
A technology of oblique phase lead tungstate and oblique phase tungstic acid, which is applied in chemical instruments and methods, tungsten compounds, inorganic chemistry, etc., can solve the problem of high market price of dextran sulfate-40, poor industrial production efficiency, and preparation process Complexity and other issues, to achieve the effect of easy control and enlarged production, cheap equipment, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] a) Dissolving lead nitrate in water to prepare 50 mL of lead nitrate solution with a lead nitrate concentration of 0.0045 mol / L;
[0034] b) Sodium tungstate is dissolved in water to prepare 50mL sodium tungstate aqueous solution with a sodium tungstate concentration of 0.009mol / L;
[0035] c) Add the aqueous solution of lead nitrate prepared in step a) dropwise to the aqueous solution of sodium tungstate prepared in step b) under stirring, and continue stirring for 10 minutes after dropping to obtain a reaction system;
[0036] The molar ratio of lead nitrate in lead nitrate aqueous solution to sodium tungstate in sodium tungstate aqueous solution is 1:2;
[0037] d) After aging the reaction system in step c) at 25°C for 72h, wash it twice with water (20mL each time), then wash once with 20mL ethanol, perform centrifugation after each wash, and then dry at 60°C for 6h, Obtain monoclinic lead tungstate.
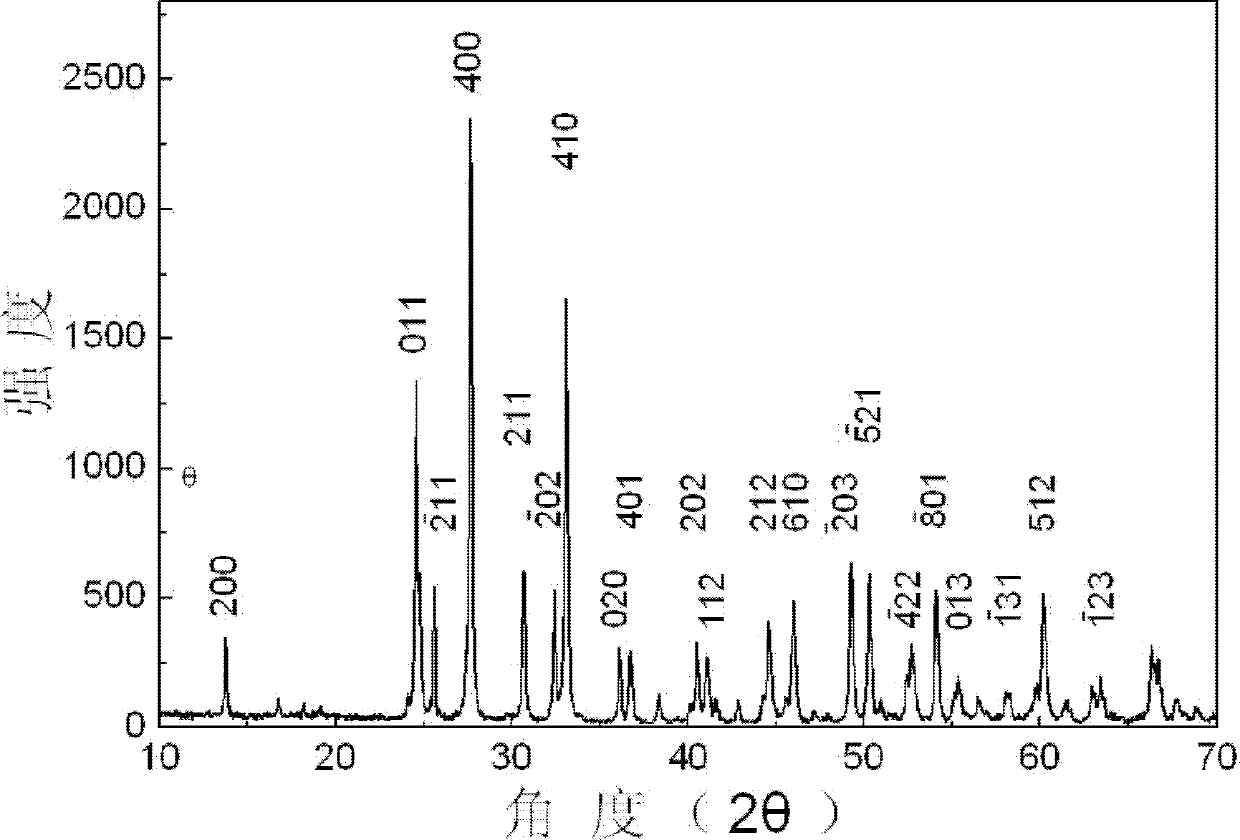
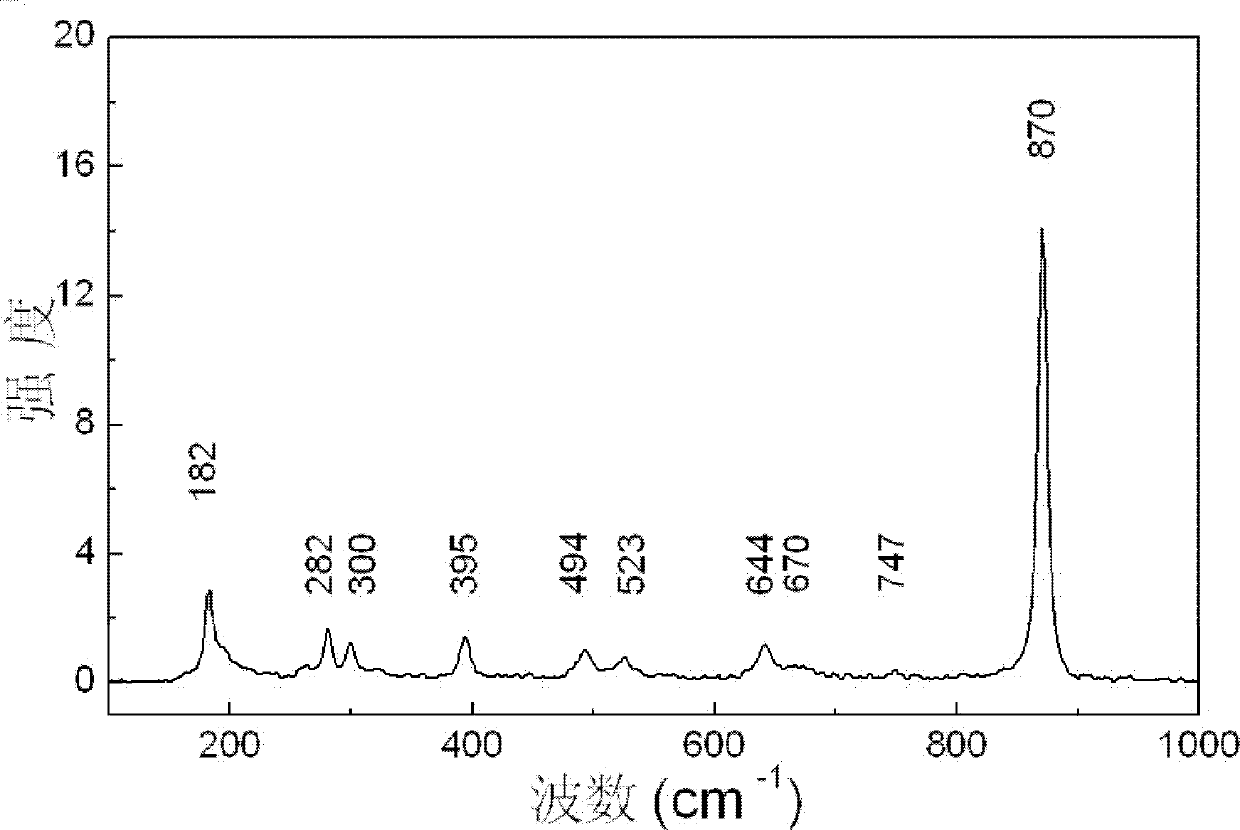
[0038] The X-ray diffraction spectrogram of the monoclinic phas...
Embodiment 2
[0043] a) Dissolving lead nitrate in water to prepare 50 mL of lead nitrate solution with a lead nitrate concentration of 0.0045 mol / L;
[0044] b) Sodium tungstate is dissolved in water to prepare 50mL sodium tungstate aqueous solution with a concentration of 0.0068mol / L;
[0045] c) Add the aqueous solution of lead nitrate prepared in step a) dropwise to the aqueous solution of sodium tungstate prepared in step b) under stirring, and continue stirring for 10 minutes after dropping to obtain a reaction system;
[0046] The molar ratio of lead nitrate in lead nitrate aqueous solution to sodium tungstate in sodium tungstate aqueous solution is 1:1.51;
[0047] d) After aging the reaction system in step c) at 25°C for 72h, wash it twice with water (20mL each time), then wash once with 20mL ethanol, perform centrifugation after each wash, and then dry at 60°C for 6h, Obtain monoclinic lead tungstate.
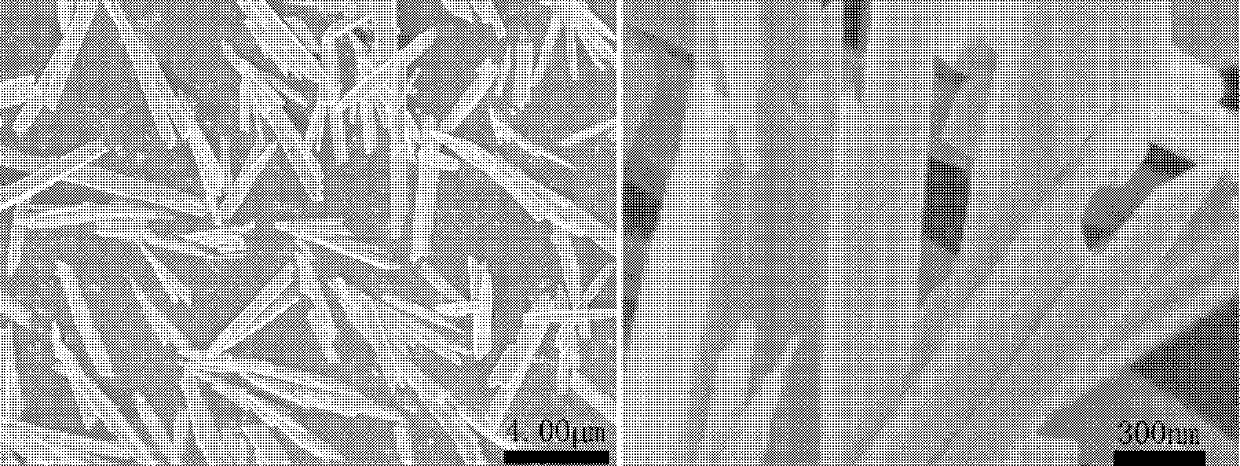
[0048] The scanning electron micrograph of the monoclinic phase lead tungsta...
Embodiment 3
[0050] a) Dissolving lead nitrate in water to prepare 50 mL of lead nitrate solution with a lead nitrate concentration of 0.0045 mol / L;
[0051] b) Sodium tungstate is dissolved in water to prepare 50mL sodium tungstate aqueous solution with a concentration of 0.0056mol / L;
[0052] c) Add the aqueous solution of lead nitrate prepared in step a) dropwise to the aqueous solution of sodium tungstate prepared in step b) under stirring, and continue stirring for 10 minutes after dropping to obtain a reaction system;
[0053] The molar ratio of lead nitrate in the lead nitrate aqueous solution to sodium tungstate in the sodium tungstate aqueous solution is 1:1.24;
[0054] d) After aging the reaction system in step c) at 25°C for 72h, wash it twice with water (20mL each time), then wash once with 20mL ethanol, perform centrifugation after each wash, and then dry at 60°C for 6h, Obtain monoclinic lead tungstate.
[0055] The scanning electron micrograph of the monoclinic phase lead...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com