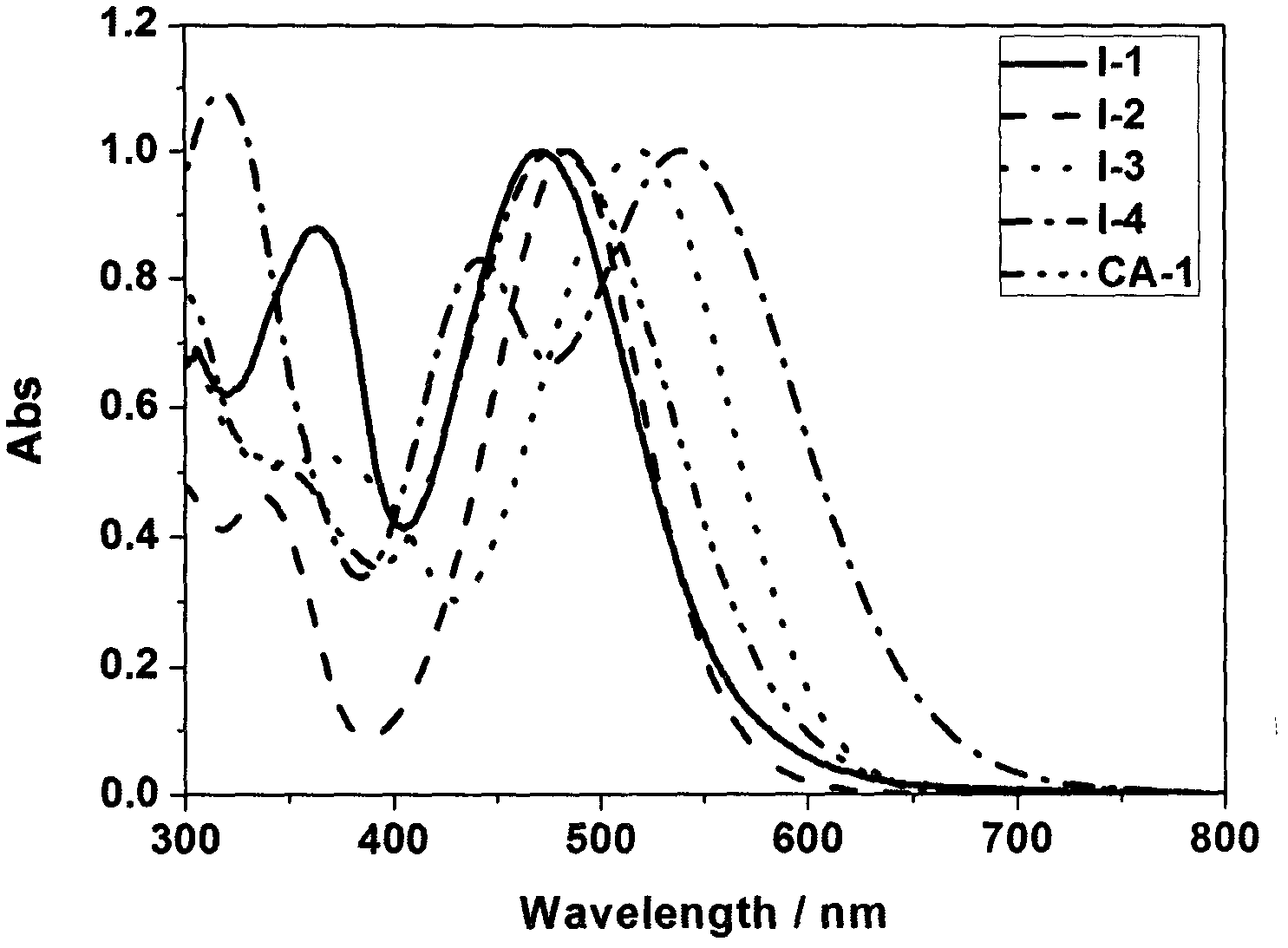
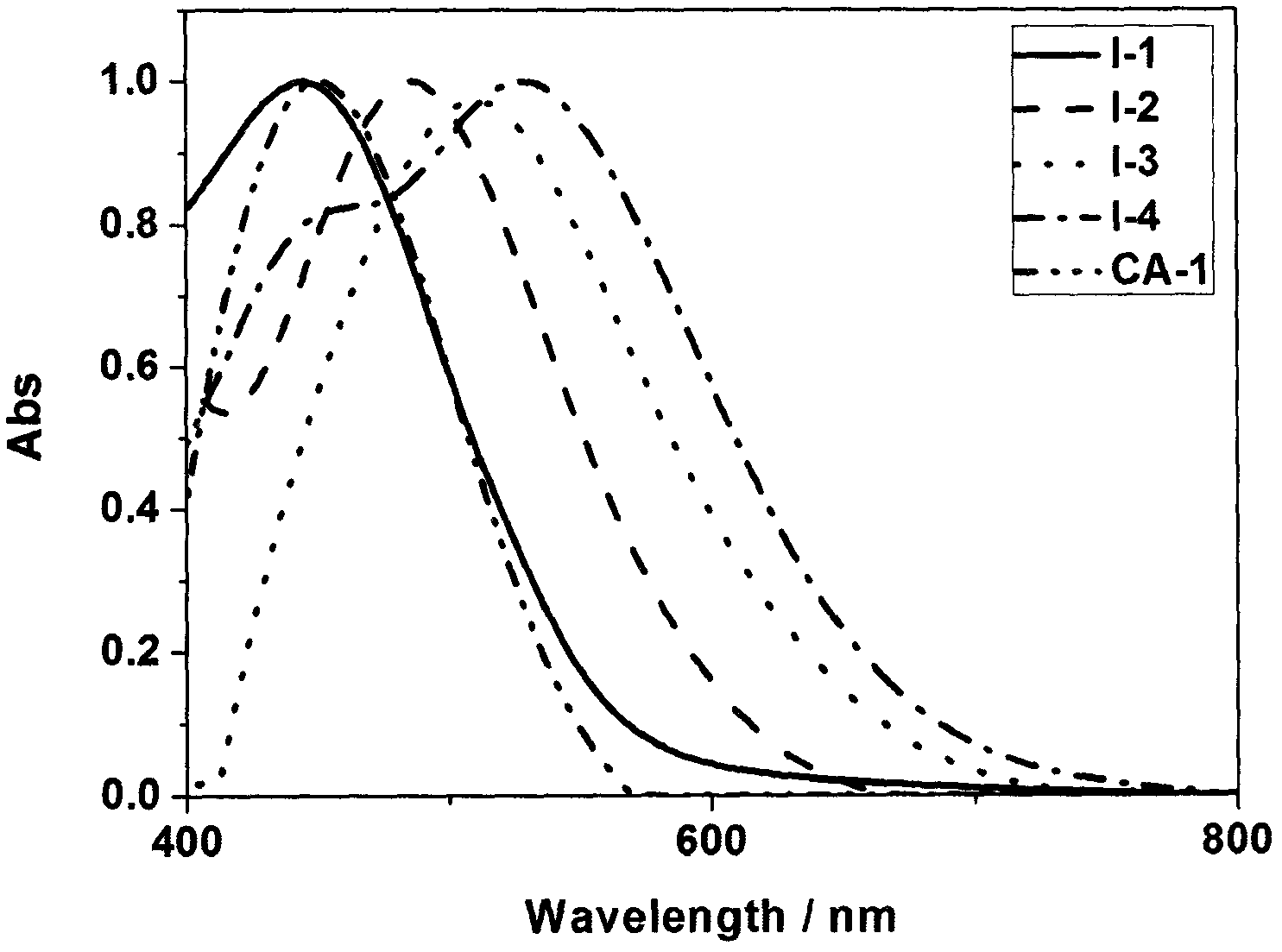
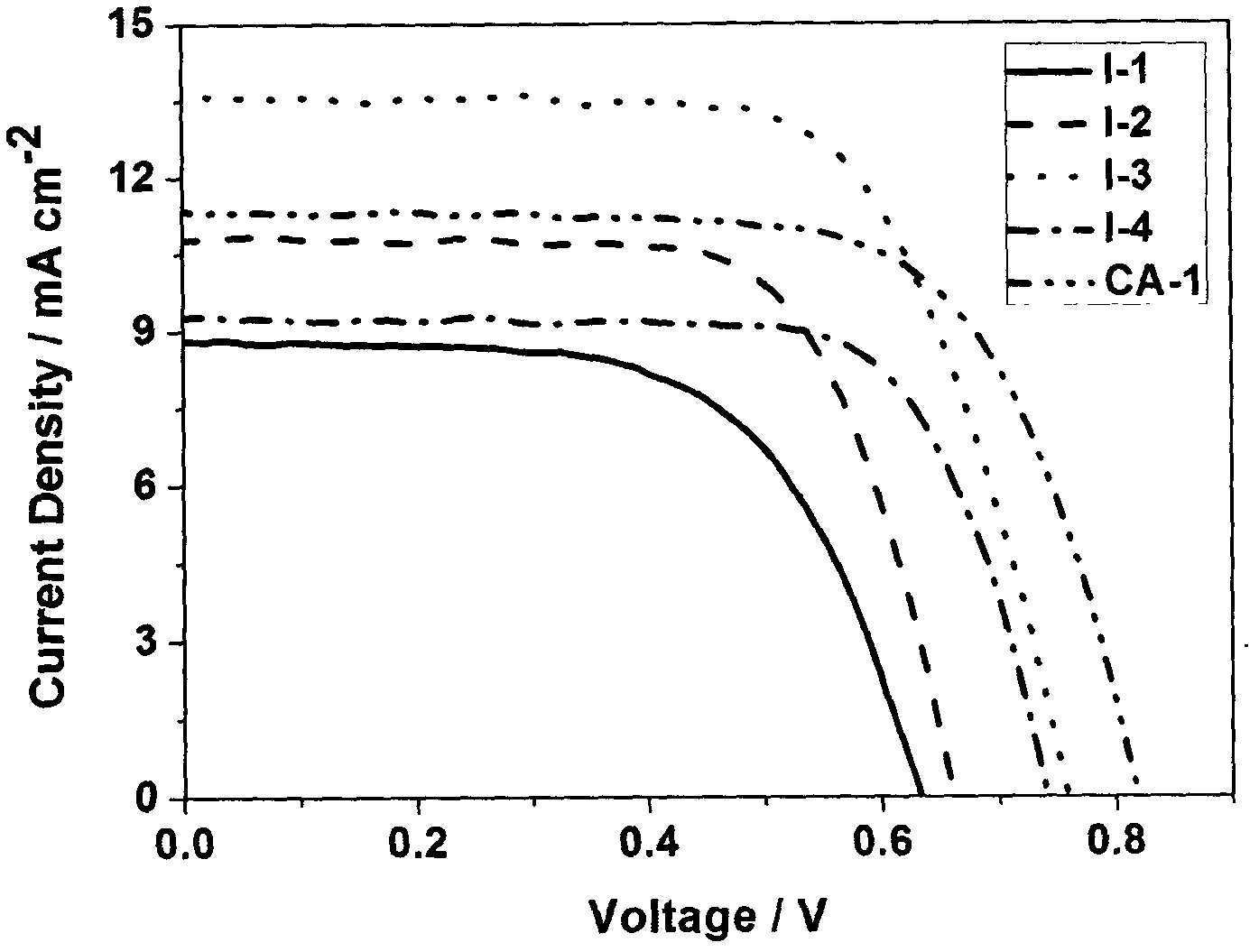
Rhodanine derivative and application thereof
A derivative and straight chain technology, applied in the field of rhodanine derivatives, can solve the problems of high price and complicated preparation process of dye sensitizers, and achieve the effects of low synthesis cost, easy preparation and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] d. Preparation of photoanode: Brush a layer of TiO on the FTO conductive glass by screen printing (0.5×0.5cm) 2 Slurry, bake at 125°C for 6 minutes, after cooling, apply another layer, bake at 125°C for 6 minutes, apply two layers in total, then slowly heat up to 450°C and bake for 15 minutes, then heat up to 500°C for 15 minutes, cool to room temperature, use 20mM TiCl 4 The aqueous solution was treated at room temperature for 12 hours, washed twice with water and ethanol respectively, baked at 450°C for 30 minutes, and soaked in the dye solution (that is, prepared by step c) after cooling. Take it out after 12 hours, wash with the solvent used to soak the dye, and blow dry.
[0039] e. Electrolyte preparation: prepare 0.05M I 2 , 0.15M LiI, 0.1M DMPII and 0.5M TBP solution 10mL.
[0040] f. Encapsulation of the battery: on TiO adsorbed with dye 2 A thermosetting adhesive tape sealing ring is pasted around the membrane, and the conductive side of the opposite elect...
Embodiment 1
[0048]
[0049] Add rhodanine (2.66g, 20mmol), malononitrile (1.32g, 20mmol), sodium acetate (1.64g, 20mmol) and absolute ethanol (60ml) into a 100ml three-necked flask, and heat to reflux for 12h under the protection of Ar. After the reaction was completed, the solvent ethanol was removed by rotary evaporation, and the remaining solid was extracted three times with dichloromethane, the organic phases were combined and dried with anhydrous magnesium sulfate, and the dichloromethane was removed by rotary evaporation, and then recrystallized with absolute ethanol to obtain 1.98 g of the product , yield 60%.
[0050] 1 H NMR (400MHz, DMSO) δ: 3.78 (s, 2H).HR-MS (EI) calculated for C 6 h 3 N 3 OS: 163.9919, found: 163.9918.
[0051]
[0052] In 250ml there-necked flask, add phenothiazine (10g, 50mmol), 5g sodium hydroxide (5g, 0.1mol) and DMSO (100ml), then add 1.6g tetra-n-butylammonium bromide (1.6g, 5mmol), react Stir at room temperature for 30 min. 1-Bromooctane (1...
Embodiment 2
[0061]
[0062] Add p-iodophenol (8.8g, 40mmol), 1-bromooctane (8ml, 44mmol), potassium carbonate (10.04g, 80mmol) and potassium iodide (6g, 40mmol) into a 250ml three-neck flask. Under the protection of Ar, 80ml of DMF was added, and the temperature was raised to 90°C and the reaction was stirred for 24h. After the reaction, the reaction solution was poured into ice water and stirred until the ice completely melted, CH 2 Cl 2 Extraction, washing with water, drying the organic phase with anhydrous sodium sulfate, spin-drying the solvent, and then performing column chromatography (PE). 9.6 g of colorless liquid was obtained with a yield of 72.3%.
[0063] 1 H NMR (400MHz, CDCl 3 )δ: 7.94(s, 2H), 6.68(s, 2H), 4.10(s, 2H), 1.73(s, 2H), 1.43(s, 2H), 1.28(d, J=19.9Hz, 8H), 0.89(s, 3H).
[0064]
[0065] In a 250ml three-necked flask, add diphenylamine (1.4g, 15mmol), 3 (25.2g, 76mmol), o-phenanthroline (2.97g, 15mmol), cuprous chloride (2.87g, 15mmol), potassium hydroxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com