Sika deer IGF (Insulin-like Growth Factor)-1 polypeptide and preparation method thereof
A technology of IGF-1 and sika deer, applied in the biological field, can solve the problems of expensive enzyme reagents, affecting protein activity, and greatly affecting the refolding of small molecular weight proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Cloning of sika deer IGF-1 mature peptide gene
[0026] The total RNA of sika deer antler tissue (from Jilin Agricultural University Experimental Deer Farm) was extracted and reverse-transcribed into double-stranded cDNA. According to the sequence of sika deer IGF-1 gene in GenBank database (accession number: HQ890468), IGF-1 mature peptide primers were designed. Upstream primer: TTC GAATTCAAC GGACCCGAGACCCTCTG, add EcoRI restriction site and base sequence encoding Asn at the 5 end; downstream primer: CGC AAGCTTCTA GGCCGCCTTGGTGG, add a Hind III restriction site and a stop codon sequence at the 5-end; use the cDNA of sika deer antler tissue as a template for PCR amplification; the PCR reaction conditions are: 94°C pre-denaturation for 5 minutes, 94°C denaturation for 30 seconds, and 55°C annealing 30s, 72°C extension for 30s for 35 cycles, and finally 72°C extension for 10min. The PCR product was recovered using a DNA gel recovery kit, and the recovered fr...
Embodiment 2
[0028] Construction and identification of embodiment 2 recombinant expression vector
[0029]Digest the pET-32a vector and the correctly sequenced pMD-IGF-I plasmid with EcoRI and Hind III, recover them separately, and connect them with T4 DNA ligase overnight at 16°C to obtain recombinant plasmids, which are transformed into Escherichia coli Rosetta competent cells Spread on LB plates and incubate overnight at 37°C. Positive clones were selected for double-enzyme digestion identification, and then sent to TaKaRa Company for sequencing after correct identification. The clone with correct sequencing was named pET-32a-IGF-1.
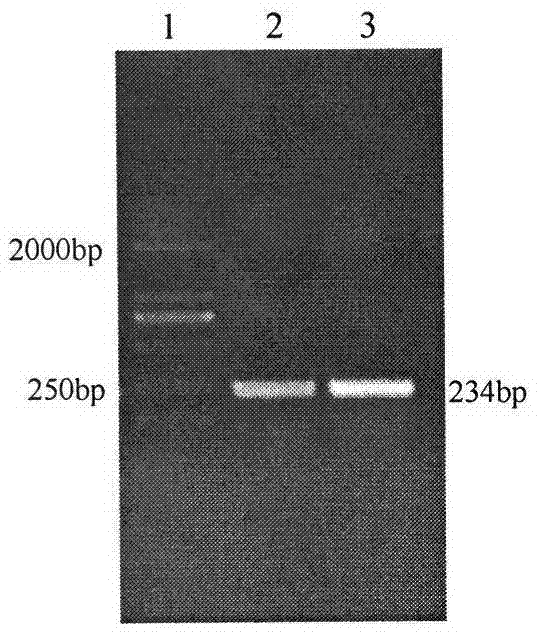
[0030] The prokaryotic expression vector pET-32a-IGF-1 was digested with EcoR I and Hind III to obtain a specific band with a size of about 234bp, which was consistent with the expected size. The results showed that the sika deer IGF-1 gene was successfully inserted into the pET-32a vector, and the prokaryotic expression vector pET32a-IGF-1 was successful...
Embodiment 3
[0037] Example 3 Cutting, purification and protein renaturation of fusion protein
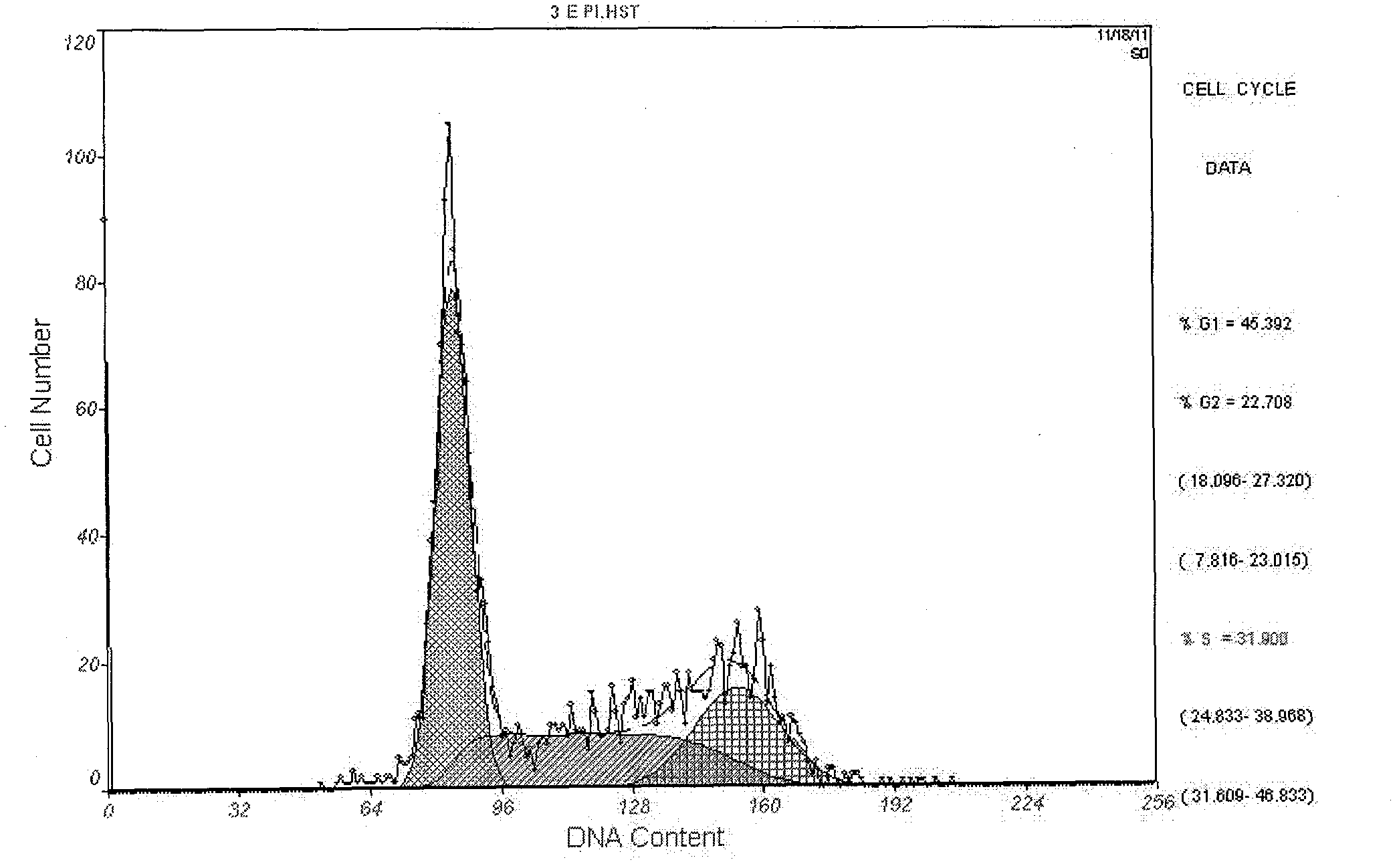
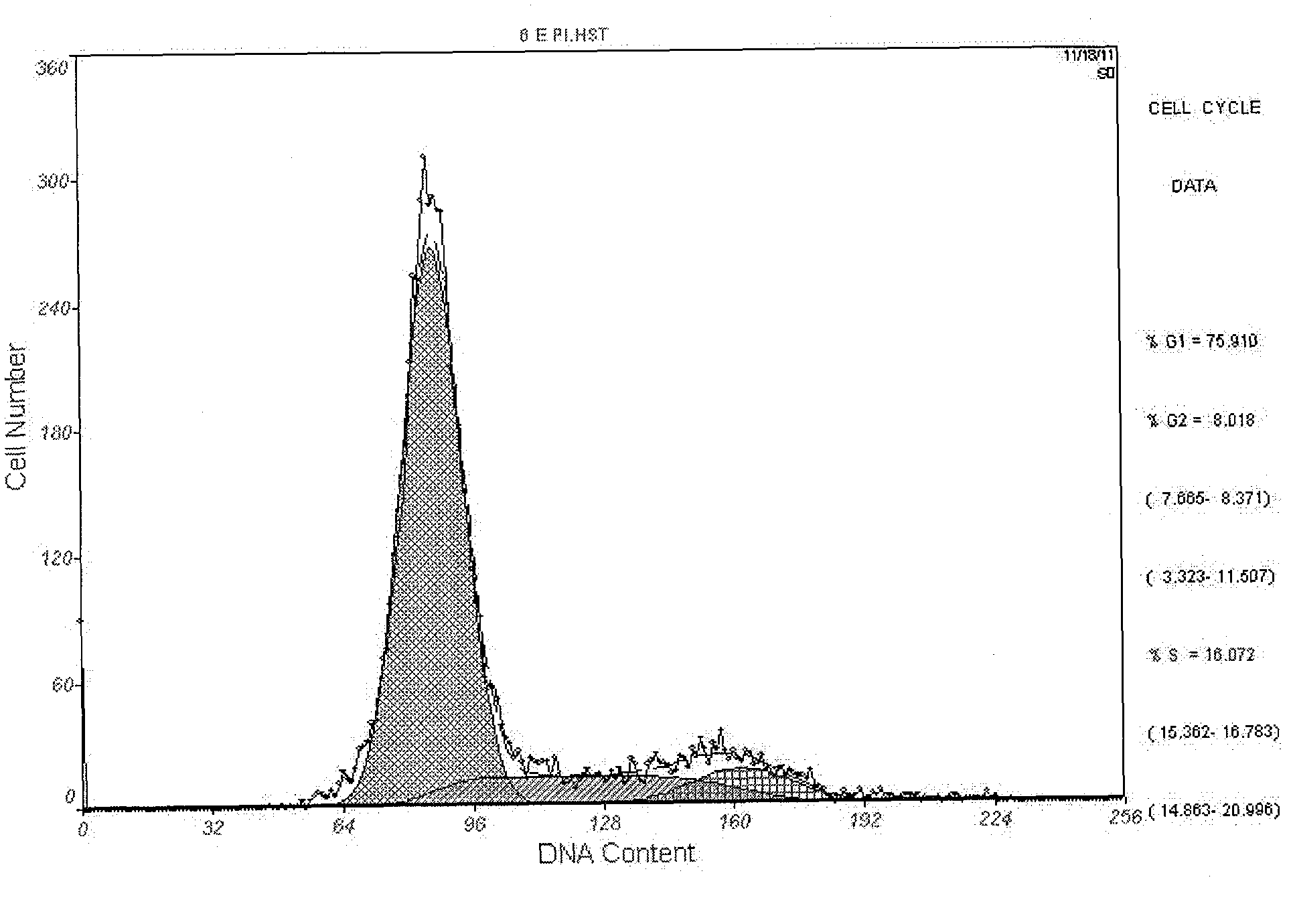
[0038] The fusion protein obtained above was diluted to a concentration of 1.0 mg / ml with hydroxylamine lysate (2mol / L hydroxylamine, 200mmol / LCHES, 8mol / L urea, pH9.5), and reacted at 45°C for 5h to cleave the fusion protein. The solution was adjusted to pH 8.0 with HCl to terminate the reaction, and after Ni-Agarose column affinity chromatography, the effluent was collected according to the 280 nm ultraviolet absorption value. The collected effluent was slowly added to the refolding solution (0.5mmol / L NaCl, 1mmol / L EDTA, 20mmol / L Tris-HCl, 1%Gly, 1mmol / L GSH, 3mmol / L GSSG, pH 8.0), so that The concentration of the recombinant protein was 0.15 mg / ml, renaturation at 4°C for 12 hours and then dialyzed to remove other impurities. SDS-PAGE electrophoresis of the recombinant protein pET32a-IGF-1 cut with hydroxylamine lysate showed that a specific protein band could be detected at about 7.5kDa; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com