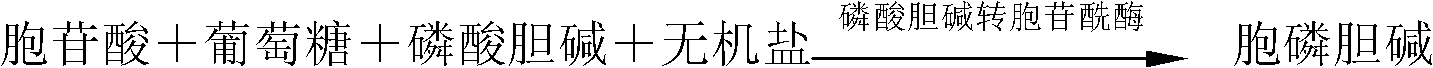Process for preparing cytidine diphosphate choline
A technology for citicoline and choline, applied in the field of biopharmaceuticals, can solve the problems of high price and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] A preparation method of citicoline based on the de novo synthesis of pyrimidine. The method includes: in the presence of a culture solution of microorganism A, or in the presence of a culture solution or treatment of microorganism B, The choline raw material is reacted for 10 to 72 hours at a reaction temperature of 20 to 38°C, and the pH is controlled to be 5 to 7.5 to accumulate citicoline.
[0017] The choline material is selected from choline, choline chloride, choline phosphate, choline iodide or choline bromide.
[0018] The microorganism A is selected from one or two of the genus Corynebacterium, Brevibacterium, Bacillus, Rseudomonas, and Saccharomyces cerevisiae Strains of the above genus. The strain used has the following characteristics:
[0019] (1) It can be metabolized to produce cytosine nucleoside products;
[0020] (2) It is resistant to structural analogues of pyrimidine and can relieve the feedback inhibition of UMP and CTP products;
[0021] (3) The cytidine...
specific Embodiment approach
[0078] The following examples further illustrate the present invention, and these examples should not be regarded as a limitation of the present invention. Unless otherwise specified, the amount of each component in the examples is mass percentage.
[0079] The sources of microorganisms in the examples are as follows:
[0080] Industrial beer yeast wet cells were purchased from Jinan Brewery.
[0081] D-Biotin was purchased from Shanghai Shenggong Biological Engineering Co., Ltd.
[0082] Corynebacterium glutamicum deposit number ATCC No. 13032, Pseudomonas cepacia deposit number ATCC No. 25416, Arthrobacter citrinum deposit number ATCC No. 11624, Corynebacterium glutamicum deposit number ATCC No. 13287, Escherichia coli deposit number ATCC No. 21148, etc. were purchased from the American Standard Biological Products Collection Center. Bacillus subtilis (Bacillus subtilis) QL28001 is preserved by the General Microbiology Center of the China Microbial Culture Collection Management Co...
Embodiment 1
[0085] A. Inoculate Bacillus subtilis QL28001 in a culture solution containing 0.5% glucose, 0.5% beef extract, 1% peptone, 0.5% yeast powder, and 0.5% sodium chloride, and cultivate it at 32°C until the sugar is exhausted to obtain subtilis Bacillus culture;
[0086] B. Inoculate the obtained culture of Bacillus subtilis QL28001 in a culture solution containing 20% glucose, 5% yeast powder, 0.5% magnesium sulfate, and 1% potassium dihydrogen phosphate at an inoculum of 5%, and cultivate at 32°C 6h; Obtain the culture solution of Bacillus subtilis QL28001;
[0087] C. The obtained culture solution of Bacillus subtilis QL28001 was continuously added with 1% choline phosphate, and the reaction was continued for 30 hours. During the process, the pH was adjusted to 6.8 with concentrated ammonia water, and the obtained culture solution contained citicoline 5.7 g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com