Fraxinellone acylhydrazone/hydrazone/ester derivatives and application in preparation of plant source insecticides
A technology of alkone acylhydrazone and derivatives, which is applied in the preparation of alkone acylhydrazone/hydrazone/ester derivatives and the application field in the preparation of botanical insecticides, which can solve the problems that have not been reported and achieve good antifeedant and poisonous activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The following is the preparation method of alkone acylhydrazone / hydrazone / ester derivatives. The method uses alkone as a raw material through allylic oxidation and ketone aldehyde reduction to obtain alkone ketone, aldehyde and alcohol, and then respectively reacts with hydrazide and benzene Hydrazine and acid react to obtain a series of alkone acylhydrazone / hydrazone / ester derivatives.
[0038] Preparation of alkone acylhydrazone / hydrazone / ester derivatives 1-31:
[0039] After dissolving a certain amount of alkone ketone / aldehyde and corresponding hydrazide or phenylhydrazine with absolute ethanol, add two drops of glacial acetic acid dropwise, heat to reflux, TLC tracking detection, after the reaction is completed, concentrate and distill off the solvent before use Preparation of silica gel sheet to separate the desired pure product;
[0040] Or add N,N'-diisopropylcarbodiimide to a certain amount of alkone alcohol, carboxylic acid and an appropriate amount of 4-dim...
Embodiment 1
[0053] 1. Product: Ashenone Acylhydrazone / Hydrazone / Ester Derivatives 1-31 (See the following for details on the physical and chemical properties of each compound)
[0054] 2. Preparation method:
[0055] The following is the synthetic route of alkone ketone:
[0056] After dissolving a certain amount of chromium oxide, pyridine and tert-butyl hydroperoxide with dichloromethane, add equimolar alkone into the above reaction solution, and after reacting for about 8 hours, add a certain amount of dichloromethane to the reaction solution methane, followed by saturated NaHSO 3 and brine washing, anhydrous Na 2 SO 4 After drying and concentrating, the pure product of alkone ketone was obtained by separating on a preparative silica gel sheet.
[0057] The physical and chemical properties of alkone ketone are as follows:
[0058] 1), white solid, melting point 141-143°C.
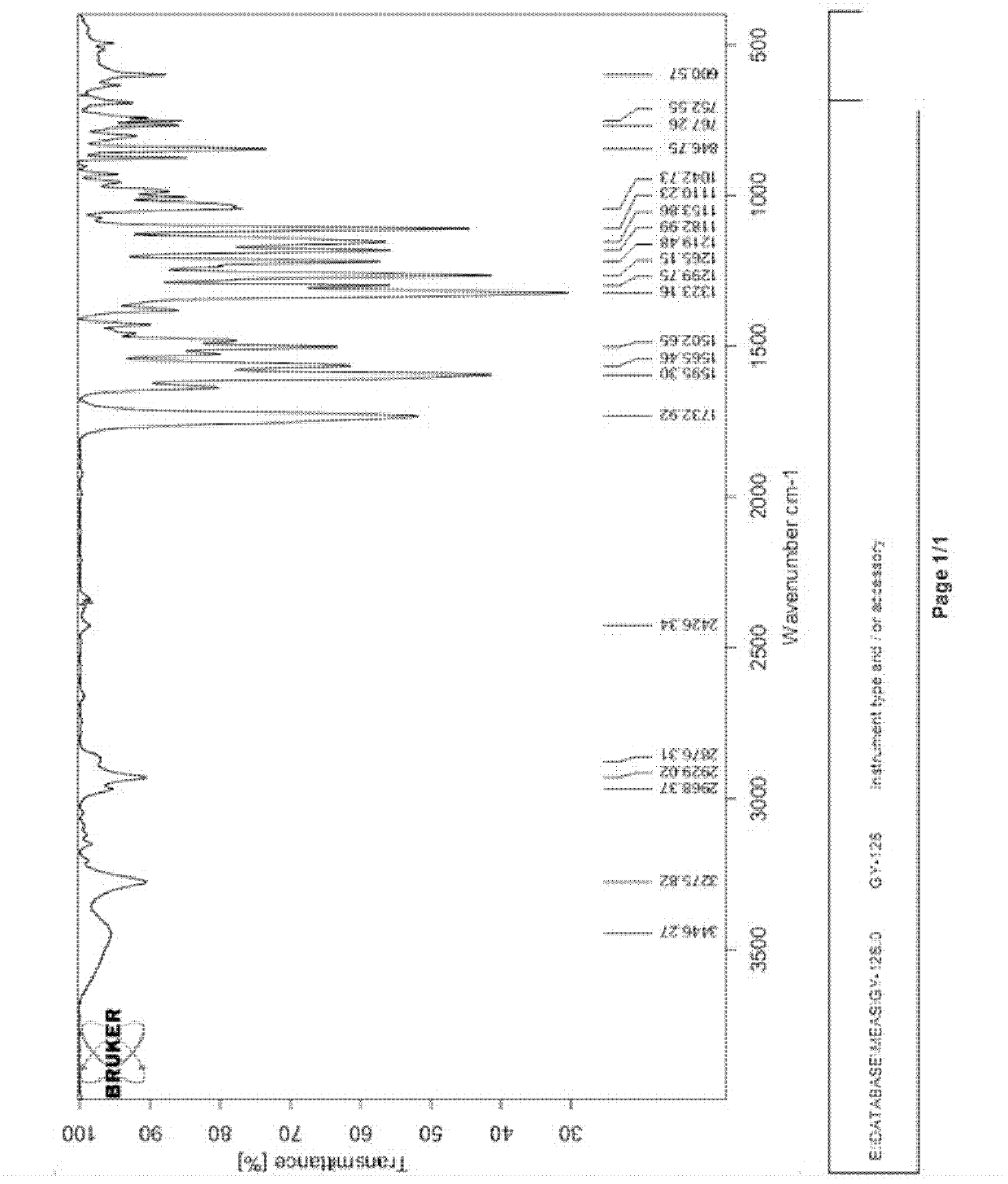
[0059] 2), the infrared spectrogram (IR) characteristic of alkone ketone:
Embodiment 2
[0254] Embodiment 2: bioassay experiment:
[0255] 1. Insects to be tested: armyworm larvae in the early 3rd instar, provided by the insect breeding room of the Pollution-free Pesticide Research Center of Northwest A&F University.
[0256] 2. Samples and reagents:
[0257] The samples are: toosendanin, arketone, arketone aldehyde, arketone ketone and compounds 1-23 prepared in the examples.
[0258] The solvent was acetone, analytically pure, from Chengdu Kelong Chemical Reagent Factory.
[0259] 3. Bioassay method:
[0260] Using the method of adding small leaf butterfly: spread a layer of filter paper on the bottom of a petri dish with a diameter of 9 cm, and add water to moisturize it. Pick 10 3rd instar prophase armyworm larvae with the same size and robustness from each dish. Weigh 5 mg of toosendanin, alkone, alkone aldehyde, alkone ketone, and compounds 1 to 23 prepared in Example 1 and add 5 ml of acetone to prepare a drug solution with a concentration of 1 mg / ml. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com