Medicinal composite tablet of pioglitazone medicine
A technology of pioglitazone hydrochloride tablets and pioglitazone hydrochloride, which is applied in the direction of drug combination, pill delivery, and pharmaceutical formulations, can solve the problems of low bioavailability of pioglitazone hydrochloride, poor water solubility of pioglitazone hydrochloride, and prolonged drug effect period, and achieve effective Conducive to the body's absorption, improve the curative effect and reduce the effect of metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The research of embodiment 1 pioglitazone hydrochloride tablet release behavior
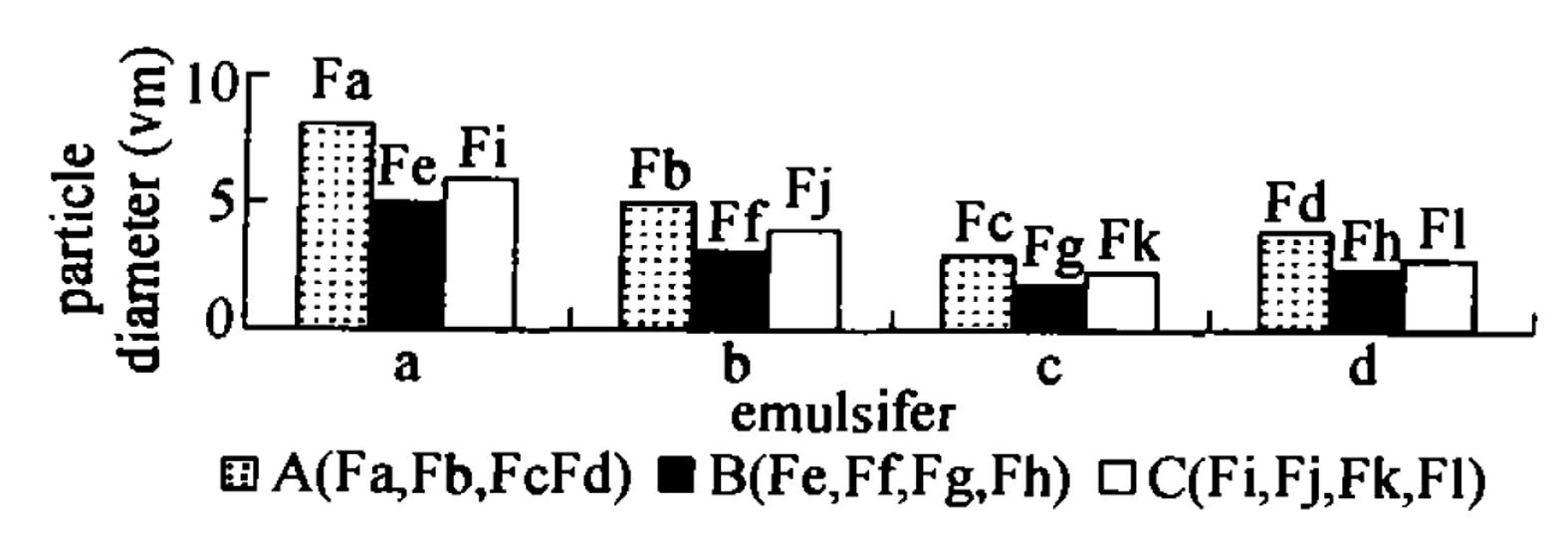
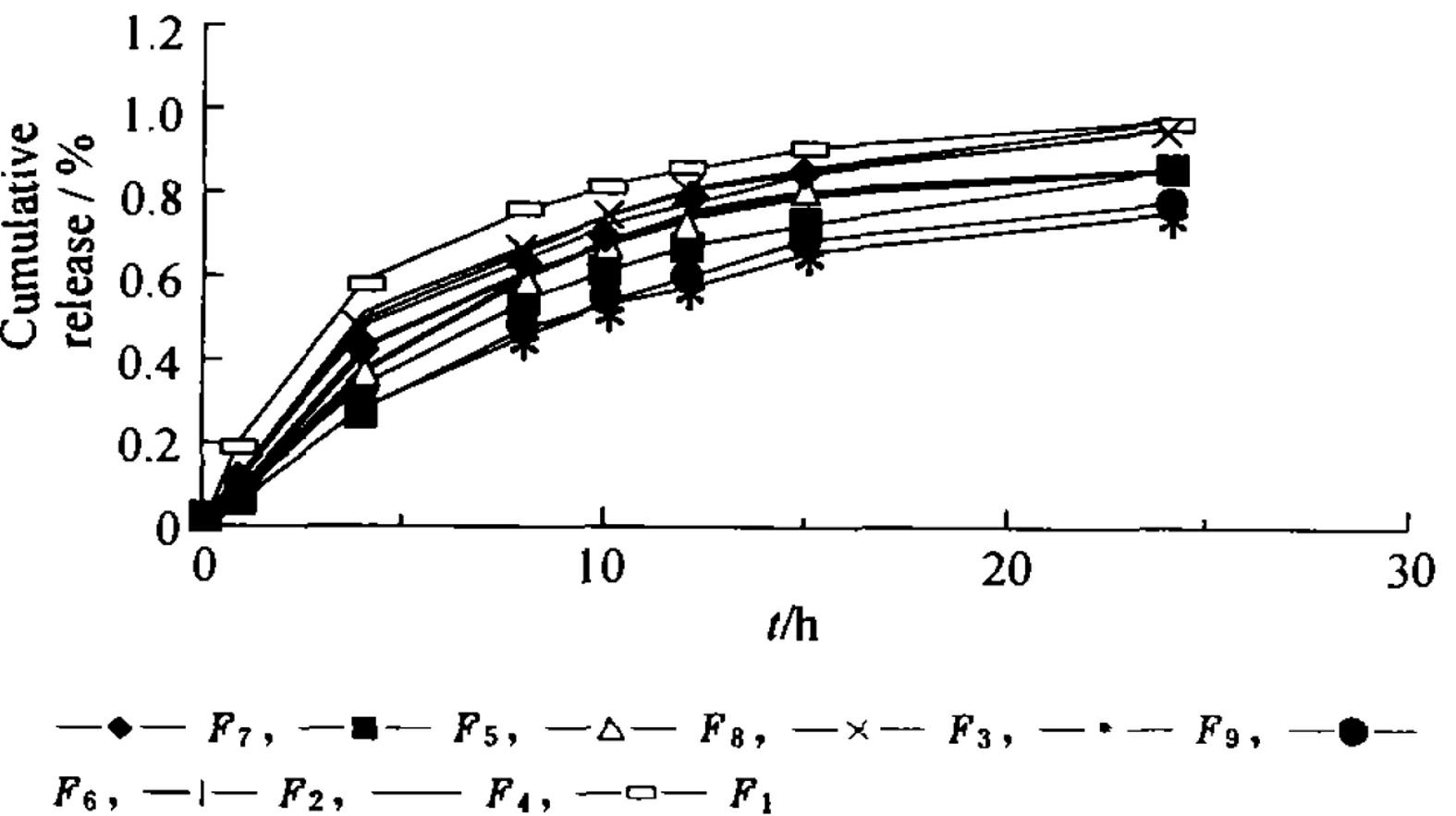
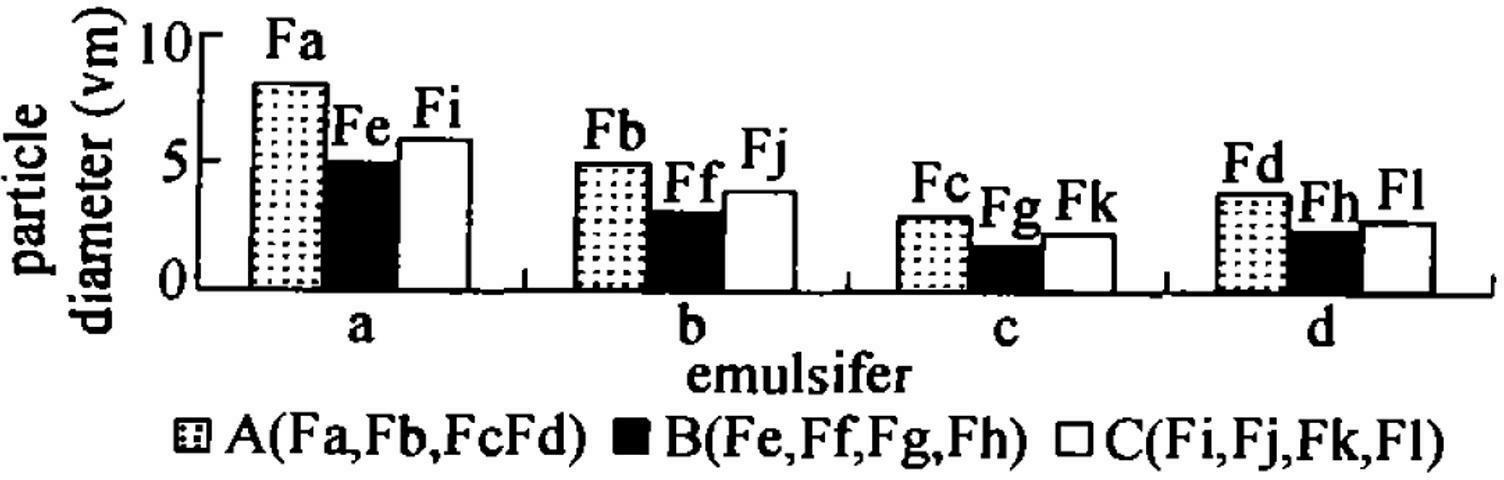
[0019] Pioglitazone hydrochloride (PGH) is a thiazolidinone hypoglycemic agent that acts on peroxisome proliferator-activated receptor-(PPAR-) and increases the sensitivity of insulin receptors. It is mainly used for the treatment of type 2 diabetes. Its efficacy is correlated with the dosage, and it has the characteristic of once-a-day administration. PGH not only has less adverse reactions, but also does not cause hypoglycemia. Using Tween-80 and phosphatidylcholine as emulsifiers, using hydroxypropyl methylcellulose (HPMC) and carbopol resin (carbopol) as skeleton materials, the self-emulsifying hydrophilic Gel extended-release tablet to enhance bioavailability.
[0020] Precisely weigh the quantitative pioglitazone hydrochloride raw material and add it to the quantitative buffer saline solution, in a 37°C water bath, with electric stirring at a speed of 50r / min, and observe the appea...
Embodiment 2
[0048] The preparation of embodiment 2 pioglitazone hydrochloride tablet
[0049] The mass ratio of pioglitazone hydrochloride, oil phase, emulsifier and emulsifier is 1:2-25:1-10:0.2-5.
[0050] According to the research conclusion described in Example 1, prepare pioglitazone hydrochloride tablet, take by weighing pioglitazone hydrochloride 15g, castor oil 30g, phosphatidylcholine 10g, soil temperature-80 5g, ethanol 3g, be prepared into self-emulsifying drug release part; Take HPMC 2.72g, carbopol 10.88g. Mix the drug and auxiliary materials evenly, use absolute ethanol to make soft material, granulate with a 24-mesh stainless steel sieve, dry at 45°C, and make 8.5mm shallow concave punched tablets with a hardness of 7±0.5kg. Obtain pioglitazone hydrochloride tablet drug group I.
[0051] Using the same method as above, weigh 15 g of pioglitazone hydrochloride, 45 g of castor oil, 30 g of phosphatidylcholine, 30 g of Tween-80, 15 g of ethanol, 8.1 g of HPMC, and 24.3 g of ...
Embodiment 3
[0053] Example 3 Study on the Tissue Distribution of Pioglitazone Hydrochloride Self-emulsifying Drug Release Part in Liver and Kidney
[0054] 1. Test material
[0055] Pioglitazone hydrochloride self-emulsification drug release part (drug group I prepared according to the method of Example 2 of the present invention, was diluted with physiological saline according to the required concentration during the test, and each bottle of 100ml contained 2.5g of pioglitazone hydrochloride), oral gavage of pioglitazone hydrochloride solution (Use 5% ethanol solution to prepare pioglitazone hydrochloride into a 6mg / ml solution). SD rats (male, mass 200±20g).
[0056] 2. Instrument and chromatographic conditions
[0057] Waters2695 HPLC, Waters2996 UV detector; Symmetry shield C18 column (4.6mm×250mm, 5μm); mobile phase acetonitrile: water (35:65); flow rate 0.5ml / min, column temperature 30°C, UV detection The wavelength is 269nm, and the injection volume is 20μl.
[0058] 3. Methods...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com