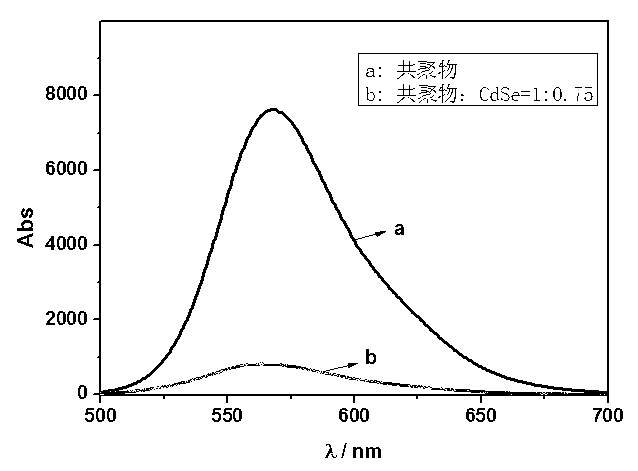
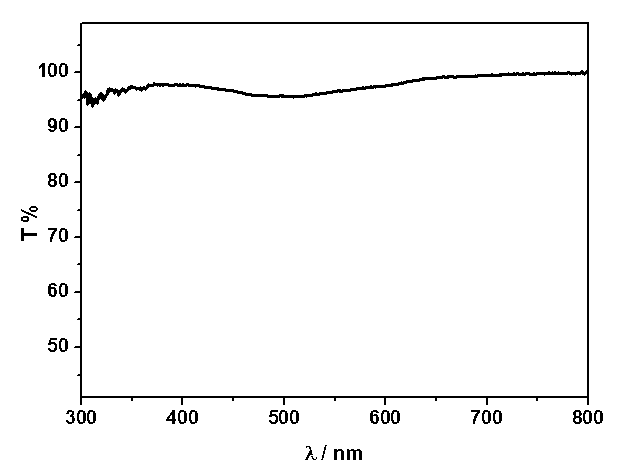
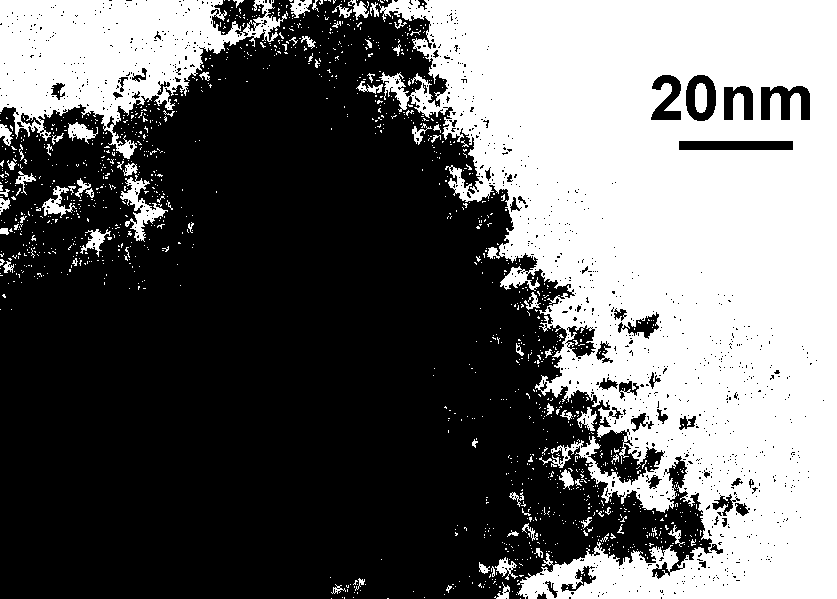Preparation method for transparent organic and inorganic hybridization heterojunction material
A heterojunction and hybrid technology, which is applied in the field of transparent organic-inorganic hybrid heterojunction materials and their preparation, can solve the problems of adverse effects on photophysical properties, solubility, harsh reaction conditions, and easy aggregation of inorganic nanocrystals. Effects of improving dispersion stability and interface compatibility, increasing carrier life, and improving absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 3-hexylthiophene-pyridine copolymer and cadmium selenide (CdSe) hybrid heterojunction material
[0026] The preparation of step 1,3-hexylthiophene-pyridine copolymer:
[0027] Under the protection of nitrogen, add 2mmol magnesium wire, 1mmol 2,5-dibromo-3-hexylthiophene and 40ml anhydrous tetrahydrofuran into a dry three-necked flask, and react under reflux for 3h to prepare 3-hexylthiophene dimagnesium bromide. In the above reaction, 1 mmol of magnesium and 1 mmol of 2,5-dibromo-3-hexylthiophene can be fed to prepare 3-hexylthiophene magnesium bromide. Its reaction formula is as follows respectively:
[0028]
[0029] Mix 1 mmol 3-hexylthiophene magnesium bromide, 1 mmol 2,5-dibromopyridine and 1 mmol 3-hexylthiophene magnesium bromide, and add 0.035 mmol 1,3-bisdiphenylphosphinopropane nickel dichloride (Ni (dppp)Cl 2 ) catalyst for coupling reaction, the coupling reaction formula is as follows:
[0030]
[0031] Stir at room temper...
Embodiment 2
[0039] Example 2: Preparation of 3-hexylthiophene-pyridine copolymer and cadmium sulfide (CdS) hybrid heterojunction
[0040] The preparation of step 1,3-hexylthiophene-pyridine copolymer:
[0041] See Example 1 for the preparation of 3-hexylthiophene magnesium bromide, mix 1mmol 3-hexylthiophene magnesium bromide and 1mmol 2,5-dibromopyridine, and add 0.02mmol Ni(dppp)Cl at the same time 2 Catalyst carries out coupling reaction, and its reaction formula is as follows:
[0042] Stir at room temperature for 6 hours, then pour the above reaction solution into 40ml of methanol at room temperature to settle, centrifuge, and vacuum-dry the obtained solid at 40-50°C, extract with methanol, n-hexane, and chloroform in sequence, and then rotary evaporate to obtain reddish-brown 3 -hexylthiophene-pyridine copolymer, yield 60%.
[0043] Step 2, preparation of cadmium sulfide (CdS) nanocrystals:
[0044] Weigh 0.5mmol of CdO and 5mmol of sulfur powder into a three-necked bottle, add...
Embodiment 3
[0047] Example 3: Preparation of 3-hexylthiophene-pyridine copolymer and zinc oxide (ZnO) hybrid heterojunction
[0048] The preparation of step 1,3-hexylthiophene-pyridine copolymer:
[0049] Under the protection of nitrogen, add 2.5mmol of 2,5-dibromo-3-hexylthiophene, 5.0mmol of isopropylmagnesium chloride Grignard reagent and 50ml of anhydrous tetrahydrofuran into a dry three-necked flask, and react at 0°C for 1h to obtain 3- Hexylthiophene Magnesium Chloride and 3-Hexylthiophene Magnesium Chloride. Its reaction formula is as follows:
[0050]
[0051] Add 1.0mmol of 2,5-dibromopyridine to the above reaction system, then add 0.035mmol of Ni(dppp)Cl 2 Catalyst carries out coupling reaction, and its reaction formula is as follows:
[0052]
[0053] Stir at room temperature for 12 h to obtain a reaction solution. Pour the reaction solution into 50ml of methanol at room temperature to settle and centrifuge. The resulting solid is vacuum-dried at 40-50°C, extracted wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com