Clostridium beijerinckii and method for preparing biological butanol through fermentation of xylose residue serving as raw material thereof
A technology of Clostridium beijerinckii and xylose residues, applied in microorganism-based methods, biochemical equipment and methods, fermentation and other directions to improve sugar production and solvent production, solve bacterial strain capacity and raw material shortages, and reduce inhibitors Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: This example illustrates the method of subjecting the original strain Clostridium beijerinckii NCIMB 8052 to ethyl methanesulfonate (EMS) mutagenesis.
[0051] The original strain Clostridium beijerinckii C. beijerinckii NCIMB 8052 was purchased from the British National Collection of Industrial, Marine and Food Cultures (NCIMB), and the method for EMS mutagenesis was as follows:
[0052] The original strain Clostridium beijerinckii NCIMB 8052 was activated and cultured at 33-37°C for 12-18 hours to obtain vigorous growth and thick bacteria liquid; take 1 mL of fresh cultured bacteria liquid, centrifuge to collect the bacteria, and use Wash the cells with 50mM sterile phosphate buffer (pH 7.0) for 3 times, then add to the seed medium containing 0.5-2% EMS, treat for 10-60 minutes, discard the supernatant by centrifugation, and add 5% sodium thiosulfate The reaction was terminated; by calculating the survival rate, 1% EMS was the optimal mutagenic dose, and 20...
Embodiment example 2
[0053] Implementation Case 2: The present invention illustrates the method for screening excellent Clostridium beijerinckii.
[0054] Wherein, the medium formula used is as follows:
[0055] (1) Starch plate medium: soluble starch 5g / L, glucose 5g / L, yeast powder 1g / L, dipotassium hydrogen phosphate 0.5g / L, potassium dihydrogen phosphate 0.5g / L, ammonium acetate 2.2g / L, Magnesium sulfate heptahydrate 0.2g / L, manganese sulfate monohydrate 0.01g / L, ferrous sulfate heptahydrate 0.01g / L, sodium chloride 0.01g / L, p-aminobenzoic acid 0.001g / L, vitamin B 1 0.001g / L, biotin 0.0001g / L, agar 15g / L, the rest is water, pH 6.5.
[0056] (2) 2-deoxy-D-glucose plate medium: soluble starch 5g / L, 2-deoxy-D-glucose 5g / L, yeast powder 1g / L, dipotassium hydrogen phosphate 0.5g / L, potassium dihydrogen phosphate 0.5g / L, ammonium acetate 2.2g / L, magnesium sulfate heptahydrate 0.2g / L, manganese sulfate monohydrate 0.01g / L, ferrous sulfate heptahydrate 0.01g / L, sodium chloride 0.01g / L, p-amino Ben...
Embodiment 4
[0069] Example 4: This example illustrates the passage stability of the mutagenized strain Y-3.
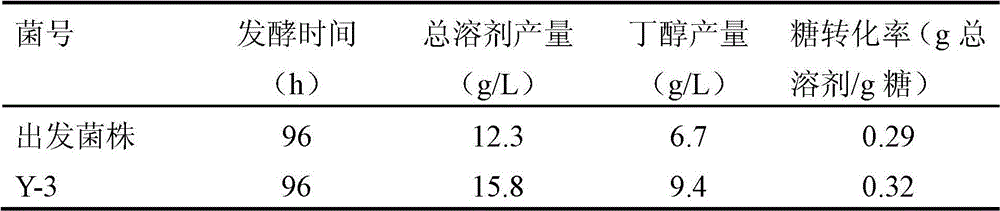
[0070] In the fermentation medium with glucose as the carbon source, the subculture stability of the mutant strain Y-3 was tested. The results showed that after 8 transfers, the amylase activity was stable, and the butanol and total solvent production were stable, which was consistent with the shake flask fermentation screening results. unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com