Pharmaceutical application of caffeic acid 3, 4-dihydroxyl phenethylester
A technology of hydroxyphenethyl ester and caffeic acid, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, antineoplastic drugs, etc., can solve problems such as hyperparathyroidism, affect bone strength, and accelerate calcium deposition, and achieve Effects of promoting osteoblast differentiation, inhibiting resorption function, and promoting its function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
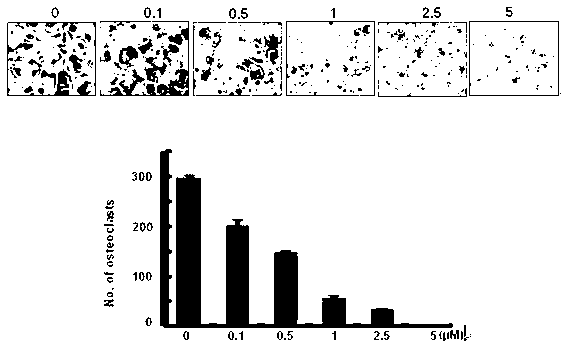
[0029] Example 1 : CADPE inhibits osteoclast precursor cell differentiation
[0030] Principle: Under the stimulation of cytokine M-CSF, bone marrow stem cells will differentiate into osteoclast precursor cells, and at the same time, under the continuous stimulation of cytokines (such as RANKL, M-CSF) Contact and fuse with each other to form multinucleated cells, and then continue to differentiate to form mature osteoclasts with a seal ring structure. The mouse osteoclast precursor cell line RAW264.7 can also differentiate into multinucleated osteoclasts under the action of RANKL cytokine. Mature osteoclasts can be adsorbed on the bone matrix and degrade the bone by analyzing protease, so as to achieve the purpose of metabolizing the bone. By detecting the formation ability of osteoclasts, the inhibitory effect of drugs on osteoclast differentiation and formation can be detected, so that the potential therapeutic effect of drugs on osteoporosis can be analyzed.
[0031] In...
Embodiment 2
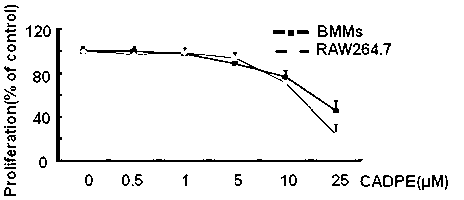
[0038] Example 2 : Cytotoxicity test of CADPE on osteoclasts
[0039] Principle: Sulforhodamine B staining is a classic technique aimed at evaluating the toxicity of living cells by quantitatively measuring the total protein synthesis rate in cells by using a protein-binding red dye, ie, colorimetry. As one of the protein stains, sulforhodamine B (SRB), also known as acid red 52 (Acid Red 52), is an anionic dye that binds to protein electrostatics and absorbs at a wavelength of 540nm Peak: The absorbance value is linearly positively correlated with the cell mass.
[0040] The cytotoxicity test method of present embodiment CADPE to osteoclast is as follows:
[0041] BMMs in 96-well plate , after overnight cell attachment, containing M-CSF (20ng / ml) - MEM culture prepared CADPE with concentrations of 0, 0.5 μM, 1 μM, 5 μM, 10 μM, 25 μM to treat the cells for 3 days, and change the medium every other day. After the experiment, the cytotoxicity of CADPE was analyzed by the ...
Embodiment 3
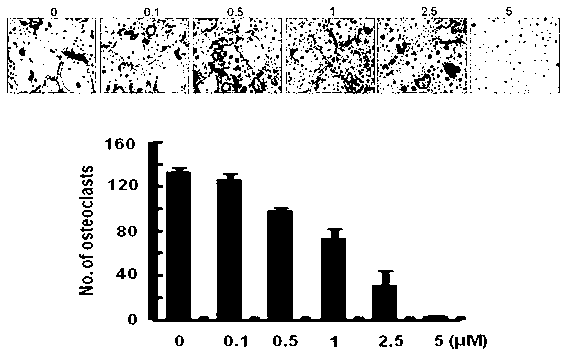
[0043] Example 3 : CADPE inhibits actin filament loop formation
[0044] Principle: When osteoclasts perform the function of resorbing bone, they will form a closed acidic environment in the local area of the surface in contact with the bone, and the formation of this environment depends on a special ring structure. The main component of this structure is Actin, so this structure is called actin ring (actin-ring). Actin ring formation is a prerequisite for bone resorption by osteoclasts. Therefore, the effect of drugs on osteoclast bone resorption can be evaluated by detecting the formation of actin ring structure.
[0045] In this embodiment, CADPE inhibits the formation of actin filament rings and the experimental method is as follows:
[0046] The precursor cells were differentiated into mature osteoclasts under the stimulation of M-CSF (20ng / ml) and RANKL (30ng / ml). Thereafter, the cells were fixed with 4% paraformaldehyde for 10 min and washed three times with PBS....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com