Preparation method for lithium iron phosphate material of positive electrode of lithium ion secondary battery
A technology of lithium iron phosphate and positive electrode materials, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of low electronic conductivity, lithium ion diffusion rate, poor conductivity of lithium iron phosphate, and high cost, and achieve easy control of process parameters, The synthesis process is easy and the material particles are uniform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
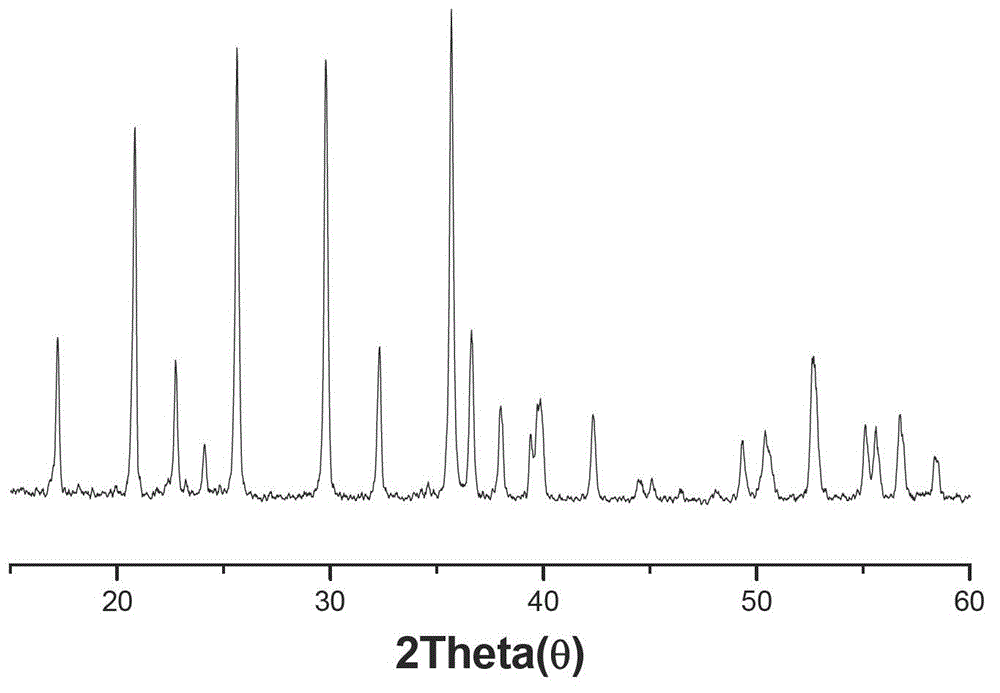
[0022] Embodiment 1, with reference to figure 1 :
[0023] Get 639 grams of lithium carbonate, 2500 grams of iron phosphate, 4.1 grams of nickel acetate, 4.12 grams of cobalt acetate tetrahydrate, 20.5 grams of ammonium molybdate, 50.4 grams of ammonium tungstate, and 414 grams of rock sugar.
[0024] Add 1452 grams of deionized water to the stirring mill, and add the above-mentioned substances to the stirring mill in turn. Stir for 3 hours.
[0025] The above mixture was diluted to a solid content of 18%, and then spray-dried.
[0026] Take the spray-dried material and place it in a reducing atmosphere furnace, sinter it in a nitrogen atmosphere at a rate of 2°C / min, first raise the temperature to 200°C, keep it at 400°C for 4 hours, keep it at 700°C for 15 hours, and then lower the temperature to obtain Lithium iron phosphate cathode material. The obtained positive electrode material is packed into a simulated battery, the diaphragm is celgard2400, the negative electrode...
Embodiment 2
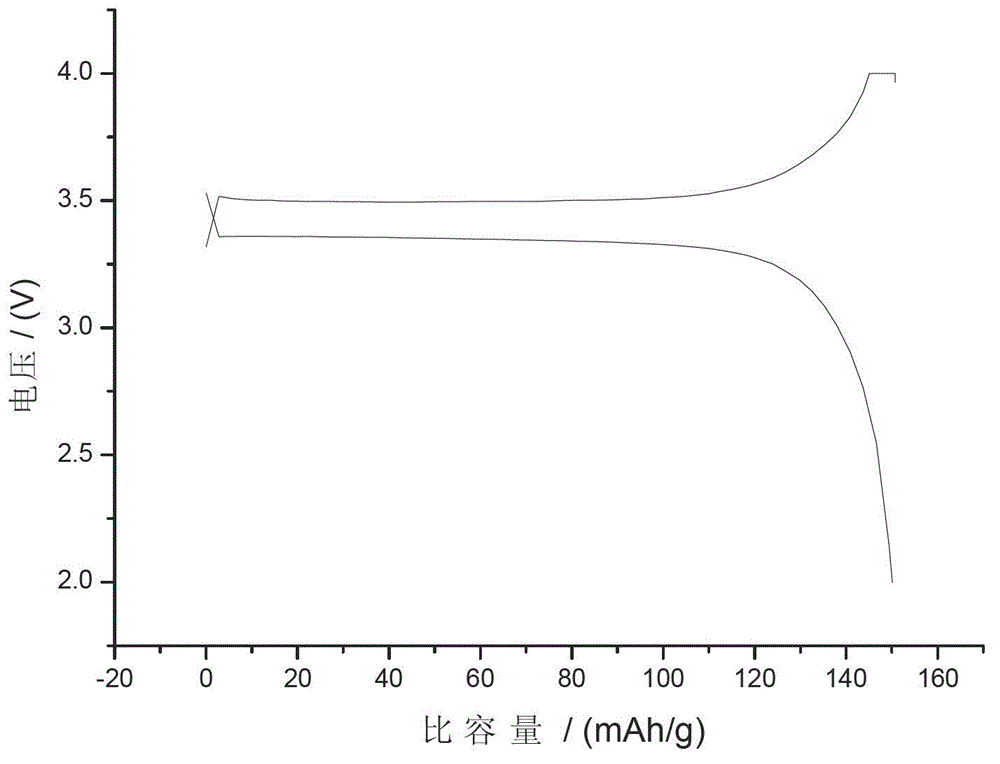
[0027] Embodiment 2, with reference to figure 2 :
[0028] Get 543 grams of lithium carbonate, 2200 grams of iron phosphate, 7.1 grams of nickel acetate, 14 grams of cobalt acetate tetrahydrate, 30.4 grams of ammonium tungstate, and 361 grams of glucose.
[0029] Add 1390 grams of deionized water to the stirring mill and add the above materials to the stirring mill in sequence. Stir for 4 hours.
[0030] The above mixture is diluted to a solid content of 20% after dilution, and then spray-dried.
[0031] Take the spray-dried material and place it in a reducing atmosphere furnace, sinter it in a nitrogen atmosphere at a rate of 2°C / min, first raise the temperature to 200°C, keep it at 400°C for 3 hours, keep it at 700°C for 15 hours, and then lower the temperature to obtain Lithium iron phosphate composite cathode material.
[0032] The obtained positive electrode material is packed into a simulated battery, the diaphragm is celgard2400, the negative electrode is metal lit...
Embodiment 3
[0033] Embodiment 3, with reference to image 3 :
[0034]Get 712 grams of lithium carbonate, 2899 grams of iron phosphate, 6.2 grams of nickel acetate, 10.3 grams of cobalt acetate tetrahydrate, 60.5 grams of ammonium molybdate, 68.2 grams of ammonium tungstate, and 457 grams of glucose.
[0035] Add 1800 grams of deionized water in the stirring mill, and add the above-mentioned substances to the stirring mill in turn. Stir for 5 hours.
[0036] The above mixture was diluted to a solid content of 23%, and then spray-dried.
[0037] Take the spray-dried material and place it in a reducing atmosphere furnace, sinter it in a nitrogen atmosphere at a rate of 2°C / min, first raise the temperature to 200°C, keep it at 400°C for 5 hours, keep it at 700°C for 12 hours, and then lower the temperature to obtain Lithium iron phosphate composite cathode material.
[0038] The obtained positive electrode material is packed into a simulated battery, the diaphragm is celgard2400, the neg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| First discharge capacity | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
| First discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com