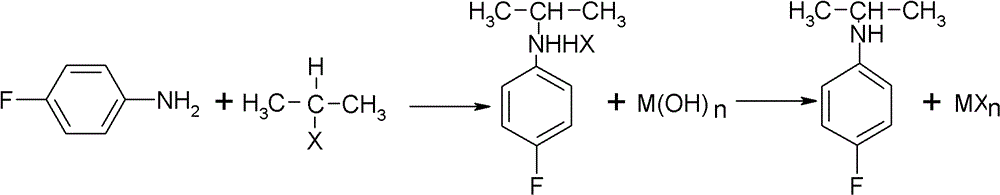
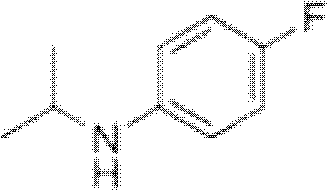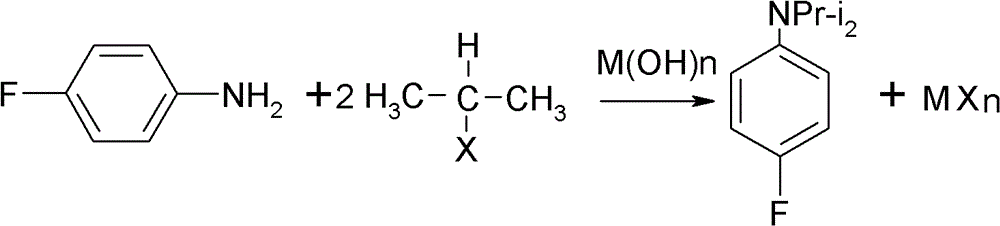Preparation method of oriented single alkylation of 4-fluorine-N-isopropyl aniline
A technology for isopropylaniline and fluoroaniline, which is applied in the field of alkylation preparation of 4-fluoro-N-isopropylaniline, can solve the problems of complex reaction operation, difficult amplification, poor product quality and the like, and achieves conversion of raw materials The effect of high rate and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a 250ml three-necked reaction flask, connect a mechanical stirrer, a condenser tube and a dropping funnel. 22g of 4-fluoroaniline, 25g of isopropyl bromide, 0.5g of PEG-400, 0.2g of tetrabutylammonium bromide, 0.2g of dodecyltrimethylammonium chloride and 0.1g of iodine were added in one portion. Dissolve 8g of sodium hydroxide in 30g of water, prepare the sodium hydroxide solution and set aside. The temperature of the system was raised to 60° C. for reflux reaction, and the reflux reaction was performed for 6 hours under the condition that the stirring speed was 200 r / min. After the reaction was cooled down to room temperature, sodium hydroxide solution was added dropwise for 30 minutes, and after the dropwise addition was completed, the reaction was terminated after being kept warm for 30 minutes. For liquid separation, add 20 ml of 0.5 mol / L dilute hydrochloric acid solution to the organic phase, stir for 30 min, and then separate the liquid to take 30 g of the o...
Embodiment 2
[0042] In a 250ml three-necked reaction flask, connect a mechanical stirrer, a condenser tube and a dropping funnel. Add 24g of 4-fluoroaniline, 34g of isopropyl iodide, 0.3g of PEG-400, 0.3g of tetrabutylammonium bromide, and 0.2g of tetramethylammonium chloride at one time. Dissolve 7g of sodium hydroxide in 30g of water, prepare the sodium hydroxide solution and set aside. The temperature of the system was raised to 90° C. for reflux reaction, and the reflux reaction was performed for 4 hours under the condition that the stirring speed was 200 r / min. After the reaction was lowered to 30°C, sodium hydroxide solution was added dropwise for 30 minutes, and the reaction was terminated after the dropwise addition was completed and kept warm for 30 minutes. For liquid separation, add 20 ml of 0.5 mol / L dilute hydrochloric acid solution to the organic phase, stir for 30 min, and then separate the liquids to obtain 31 g of the organic phase. The organic phase liquid was analyzed ...
Embodiment 3
[0044] In a 250ml three-necked reaction flask, connect a mechanical stirrer, a condenser tube and a dropping funnel. 22g of 4-fluoroaniline, 15g of isopropyl chloride, 0.2g of PEG-400, 0.2g of tetrabutylammonium chloride, 0.2g of tetramethylammonium chloride and 0.5g of potassium iodide were added in one go. Dissolve 16g of potassium hydroxide (content is 85%) in 55g of water, configure potassium hydroxide solution, set aside. The temperature of the system was raised to 35° C. for reflux reaction, and the reflux reaction was performed for 8 hours under the condition that the stirring speed was 200 r / min. After the reaction was cooled down to room temperature, potassium hydroxide solution was added dropwise for 60 minutes, and after the dropwise addition was completed, the reaction was terminated after being incubated for 40 minutes. For liquid separation, add 20 ml of 0.5 mol / L dilute hydrochloric acid solution to the organic phase, stir for 30 min, and then separate the liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com