One-dimensional copper-nitroxide free radical coordination polymer as well as preparation method and application thereof
A technology of nitroxide radicals and coordination polymers, applied in copper organic compounds, static memory, instruments, etc., to achieve high application value, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
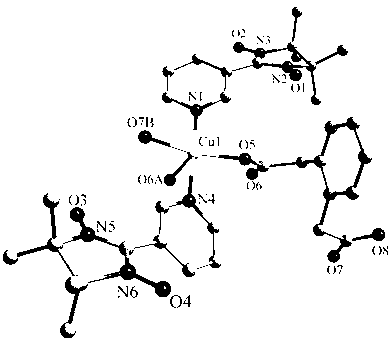
[0039] Complex {[Cu(NIT3Py) 2 (phda)] 2} n ( 1 )Synthesis:
[0040] At room temperature, copper nitrate (0.1 mmol) and meta-pyridine radical ligand (0.2 mmol) were dissolved in a mixed solvent of 10 ml of water and methanol under stirring, stirred for 0.5 hours, and then 5 ml of phthalate was added dropwise Disodium acetate (0.1 mmol) aqueous solution was stirred for 0.5 hours, and filtered to obtain a dark blue solution. The filtrate was left to stand at room temperature and slowly evaporated. After two weeks, green strip-shaped crystals suitable for X-ray analysis were obtained. The yield was 73%.
[0041] Elemental analysis (%) C 68 h 80 Cu 2 N 12 o 16 : Calculated for C 56.38, H 5.57, N 11.61; found for C 56.30, H 5.49, N 11.72. Important infrared spectral values (KBr pellets, cm -1 ): 1612s, 1396m, 1372s.
Embodiment 2
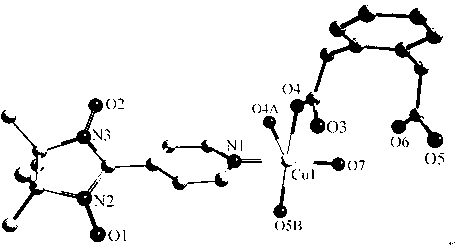
[0043] Complex {[Cu(NIT4Py)(phda)(H 2 O)] 2} n ( 2 )Synthesis:
[0044] At room temperature, copper nitrate (0.1 mmol) and p-pyridine radical ligand (0.2 mmol) were dissolved in a mixed solvent of 10 ml of water and methanol under stirring, stirred for 0.5 hours, and then 5 ml of phthalate was added dropwise Disodium acetate (0.1 mmol) aqueous solution was stirred for 0.5 hours and filtered to obtain a dark blue solution. The filtrate was left to stand at room temperature and slowly evaporated. After about ten days, dark green strip crystals suitable for X-ray analysis were obtained. The yield was 68%.
[0045] Elemental analysis (%)C 44 h 52 Cu 2 N 6 o 14 : Calcd. C 52.01, H 5.16, N 8.27; found C 52.10, H 5.25, N 8.21. Important infrared spectral values (KBr pellets, cm -1 ): 1613s, 1396m, 1372s.
Embodiment 3
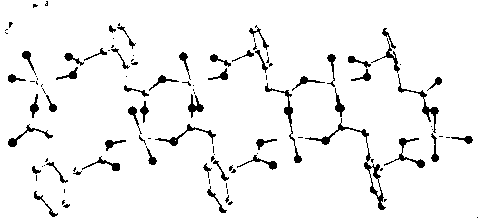
[0047] the crystal 1 with 2 Structural analysis: the selected size is 0.11 x 0.11 x 0.08 mm 3 green crystals 1 and 0.15 x 0.14 x 0.13 mm 3 dark green crystals of 2 Fixed on fiberglass. Mo-K monochromated with graphite on a Bruker APEX-II CCD X-ray diffractometer at room temperature α Rays( lambda = 0.71073 ?), with φ - ω A total of 8375 and 10637 diffraction points were collected by scanning methods. The crystal structure was solved by the direct method using the program SHELXS-97 and corrected by full matrix least squares using the program SHELXL-97. The positions of hydrogen atoms were obtained by theoretical hydrogenation, and non-hydrogen atoms were solved by direct methods. The experimental conditions, data analysis, structure analysis and correction methods and crystallographic data of the X-ray diffraction analysis of the complex are listed in Table 1;
[0048] Table 1 Complexes 1 with 2 The crystallographic data of
[0049]
[0050] Results and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com