Method for preparing hexagonal boron nitride nano composite structure
A nano-composite, boron nitride micro technology, applied in nitrogen compounds, chemical instruments and methods, nanotechnology and other directions, can solve the problems of low product purity, low product yield, coexistence of impurities, etc., to achieve high purity, large product yield, Even diameter effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
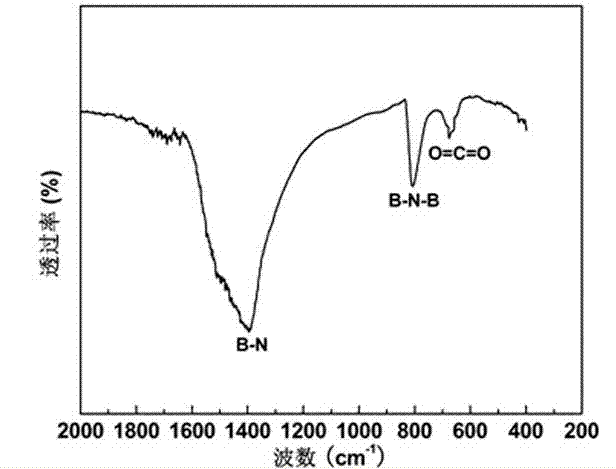
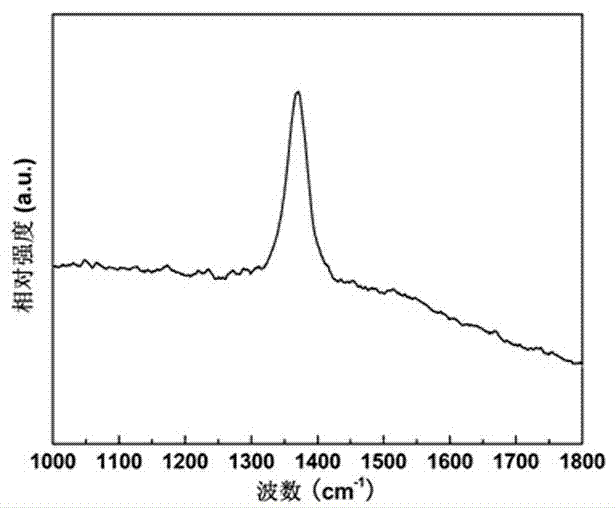
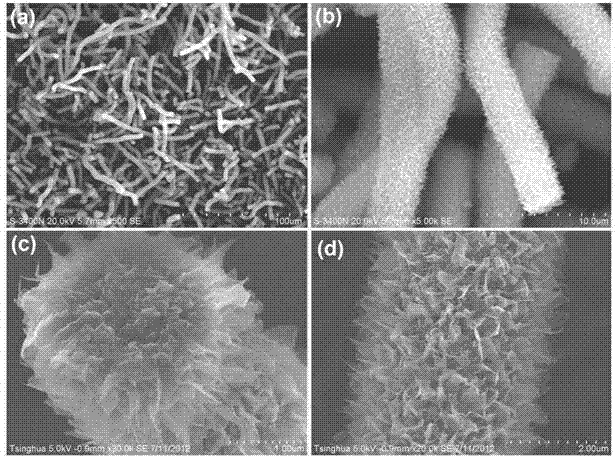
[0052] First, 2.23 grams of boron powder with a purity of 96.7% and 2.74 grams of FeCl 3 ·6H 2 O was evenly dispersed and dissolved in 5 ml of absolute ethanol, stirred for 1 hour with a magnetic stirrer to remove the ethanol solvent, and a paste mixture was obtained. Put the paste mixture into an alumina porcelain boat, and then put the alumina porcelain boat into a high-temperature tubular atmosphere furnace. Seal the high-temperature tubular atmosphere furnace, evacuate its internal space, and then introduce high-purity ammonia gas with a flow rate of 50 sccm and a purity of 95.9%, and raise the furnace temperature to 1300°C at a heating rate of 10°C / min, and keep it for 5 Hours, heat preservation ends. Then, under the protection of flowing high-purity nitrogen with a purity of more than 99.9%, the porcelain boat was naturally cooled to room temperature, and the porcelain boat was taken out to obtain a white powder, which was tested. It can be seen from the infrared spec...
Embodiment 2
[0054] First, 1.10 g of amorphous boron powder with a purity of 98% and 2.93 g of Fe 2 (SO 4 ) 3 9H 2 O was uniformly dispersed and dissolved in 10 ml of absolute ethanol, stirred for 2 hours with a magnetic stirrer to remove the ethanol solvent, and a paste mixture was obtained. Put the paste mixture into an alumina porcelain boat, and then put the alumina porcelain boat into a high-temperature tubular atmosphere furnace. Seal the high-temperature tubular atmosphere furnace, evacuate its internal space, and then introduce high-purity ammonia gas with a flow rate of 150 sccm and a purity of 95.9%, and raise the furnace temperature to 1300 °C at a heating rate of 5 °C / min, and keep it for 3 Hour. After the heat preservation is over, under the protection of flowing high-purity neon gas with a purity of more than 99.9%, the porcelain boat is naturally cooled to room temperature, and the porcelain boat is taken out to obtain a white powder, which is tested. It can be seen fro...
Embodiment 3
[0056] First, 1.20 g of amorphous boron powder with a purity of 90% and 4.04 g of Fe(NO 3 ) 3 9H 2 O was uniformly dispersed and dissolved in 15 ml of absolute ethanol, stirred for 4 hours using a magnetic stirrer to remove the ethanol solvent, and a paste mixture was obtained. Put the paste mixture into the alumina porcelain boat, and then put the alumina porcelain boat into the high-temperature tubular atmosphere furnace; seal the high-temperature tubular atmosphere furnace, evacuate its inner space, and then pass it in at a flow rate of 25 sccm, the purity Use 95.9% high-purity ammonia as protective gas, raise the furnace temperature to 1300°C at a heating rate of 15°C / min, and keep it warm for 8 hours. , the porcelain boat was naturally cooled to room temperature, and the porcelain boat was taken out to obtain a pure white powder, which was detected from the infrared spectrum. It can be seen that the obtained product is pure boron nitride; it can be further determined fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com