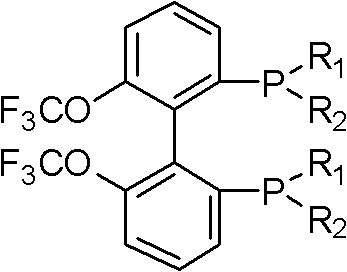
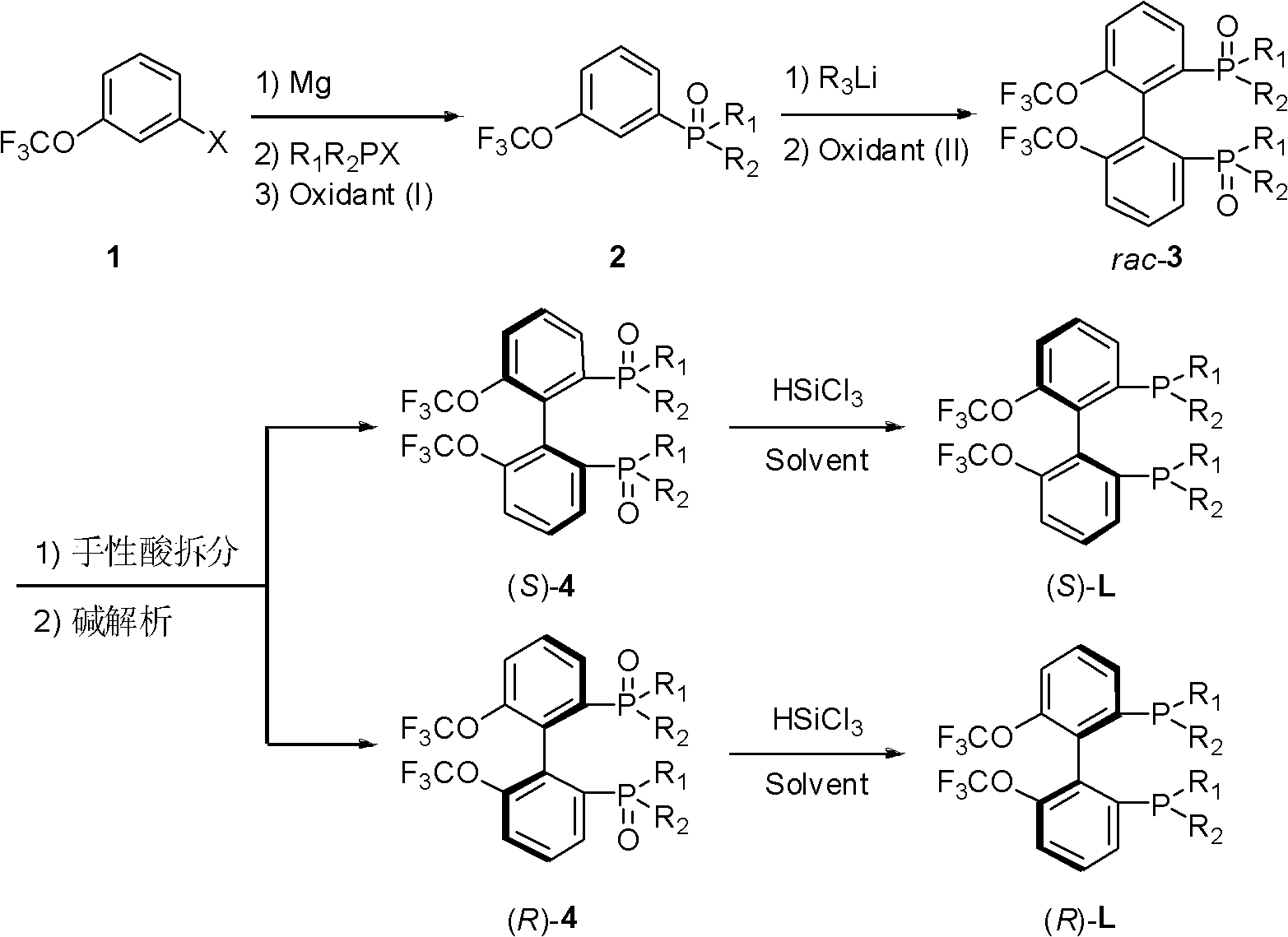
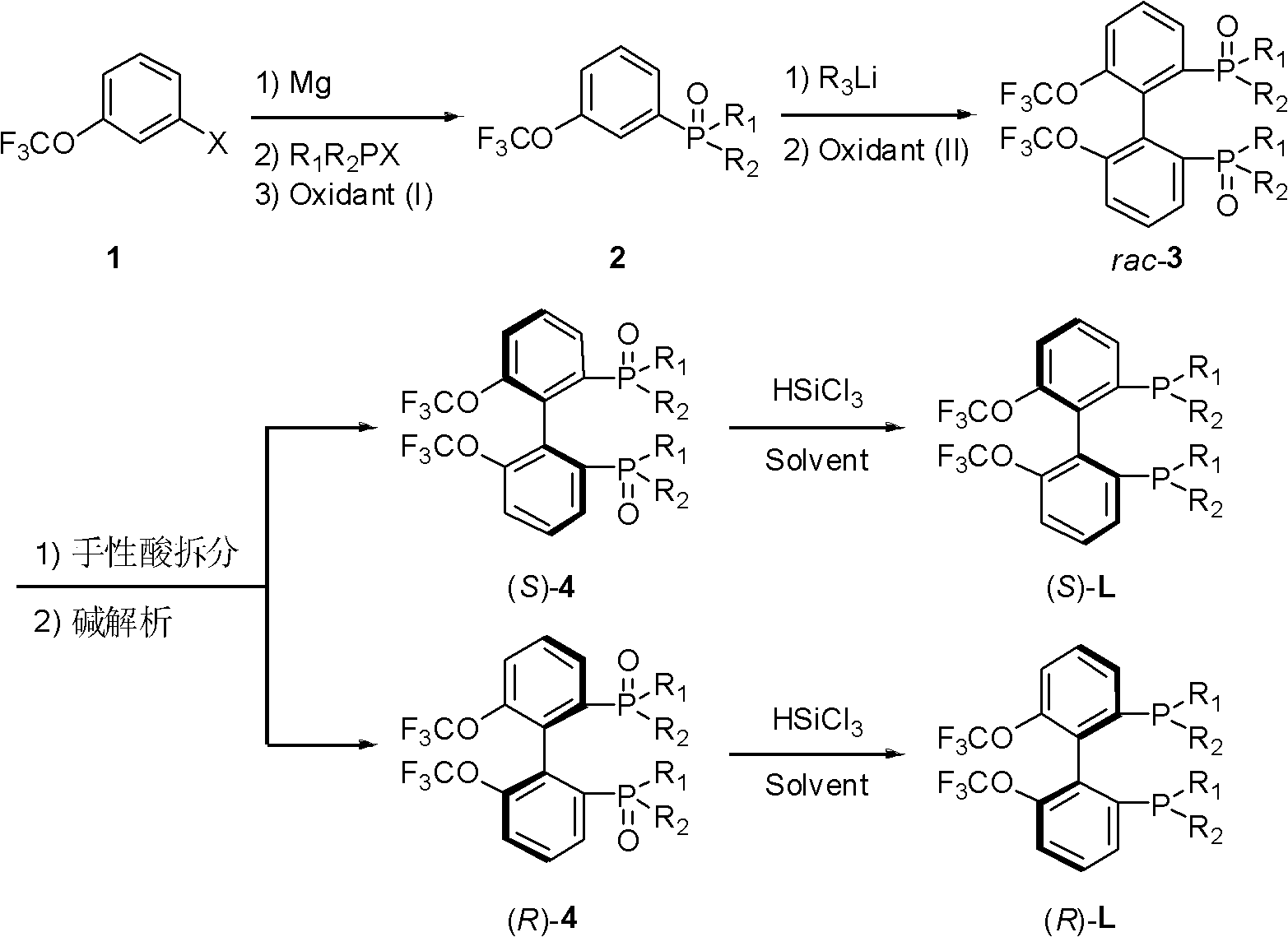
Electron-deficient axially-chiral diphosphine ligands, and preparation method thereof
A bisphosphine ligand and electron-deficient technology, which is applied in the field of design and synthesis of electron-deficient bisphosphine ligands, can solve the problems of less and less research on electronic effects, and achieve fewer reaction steps, high yield, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Synthesis of 3-trifluoromethoxyphenyldiphenylphosphine oxide (2a)
[0056]
[0057] Under nitrogen protection, magnesium chips (0.528g, 22.0mmol) and freshly distilled tetrahydrofuran (20mL) were added to a 250mL three-neck flask (fired in vacuum), and then iodine crystals (0.040g, 0.16mmol) were added. Stir, and slowly add a small amount of 3-trifluoromethoxybromobenzene 1a (3.0 mL, 20.0 mmol) in tetrahydrofuran (20 mL) dropwise at room temperature to initiate the reaction. boiling state. After the dropwise addition, the temperature was raised to 70° C. for reflux reaction for 2 h. Then, the reaction system was lowered to 0°C, and a solution of diphenylphosphorous chloride (4.1 mL, 22.0 mol) in tetrahydrofuran (15 mL) was slowly added dropwise. After the dropwise addition, the system was slowly raised to room temperature, and the reaction was continued for 3 h. The reaction system was lowered to 0°C, anhydrous methanol (10 mL) was slowly added, and sti...
Embodiment 2
[0058] Example 2: Synthesis of (±)-(6,6'-bistrifluoromethoxyphenyl)-2,2'-bis(diphenylphosphine oxide) (rac-3a)
[0059]
[0060] Under the protection of nitrogen, diisopropylamine (14.1 mL, 100.3 mmol) and freshly distilled tetrahydrofuran (100 mL) were added into a 500 mL three-necked flask (fired in vacuum). Cool the system to -78°C, add n-butyllithium (concentration in n-hexane: 2.5mol / L, 36.2mL, 90.5mmol) drop by drop under stirring, after the addition is complete, control the system temperature at -78°C to continue the reaction After 1h, a lithium diisopropylamide solution was obtained. Compound 3-trifluoromethoxyphenyldiphenylphosphine oxide 2a (27.323g, 75.4mmol) was dissolved in freshly distilled tetrahydrofuran (60mL), and added dropwise to the prepared diisopropylamino In the lithium solution, it took 20 minutes to complete the addition. After the addition was complete, the temperature of the system was controlled at -78°C to continue the reaction for 1 h. Then...
Embodiment 3
[0061] Example 3: Resolution of (±)-(6,6'-ditrifluoromethoxyphenyl)-2,2'-bis(diphenylphosphine oxide) (rac-3a)
[0062]
[0063] In a 250 mL round bottom flask was added (±)-(6,6'-ditrifluoromethoxyphenyl)-2,2'-bis(diphenylphosphine oxide)rac-3a (6.857 g, 9.5 mmol) and chloroform (27mL), the system was heated to 40°C, stirred to dissolve rac-3a, and the resolving agent D-di-p-methoxybenzoyl tartaric acid (D)-DMTA (4.568g, 9.5mmol) was dissolved A solution of ethyl acetate (41 mL) was added to the system, a large amount of white precipitates formed in the stirred solution, and the reaction was continued at 40°C for 0.5h, and then at room temperature for 1h. After filtering (recovering the filtrate), the solid was washed 3 times with a mixed solvent of chloroform and ethyl acetate (1 / 3). The solid was transferred to a round-bottomed flask, and the remaining chloroform and ethyl acetate were pumped off with an oil pump to obtain 4.447 g of the salt formed by (S)-3a and (D)-DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com